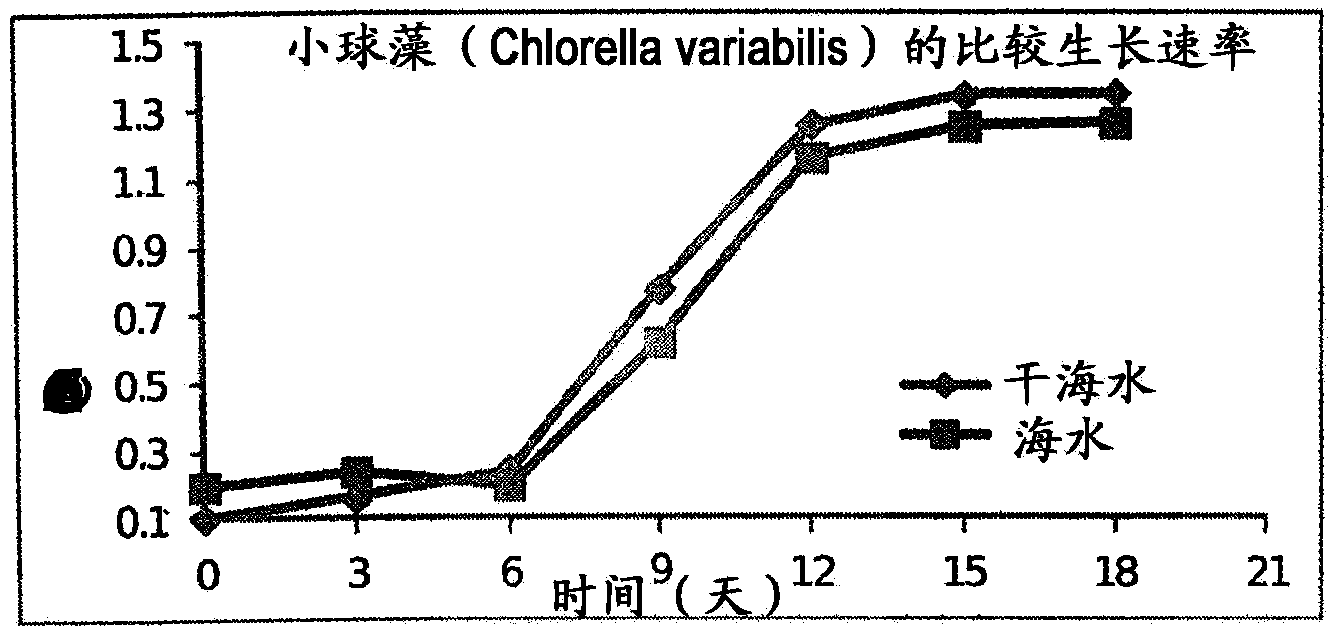
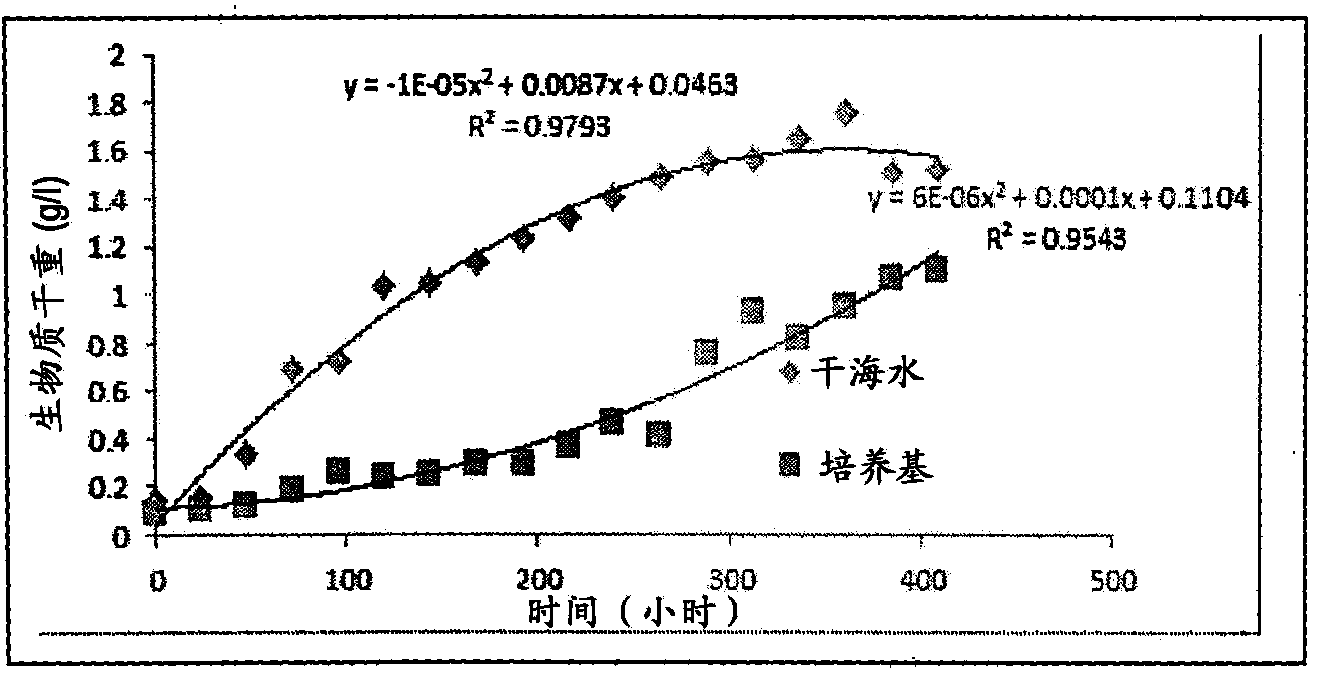
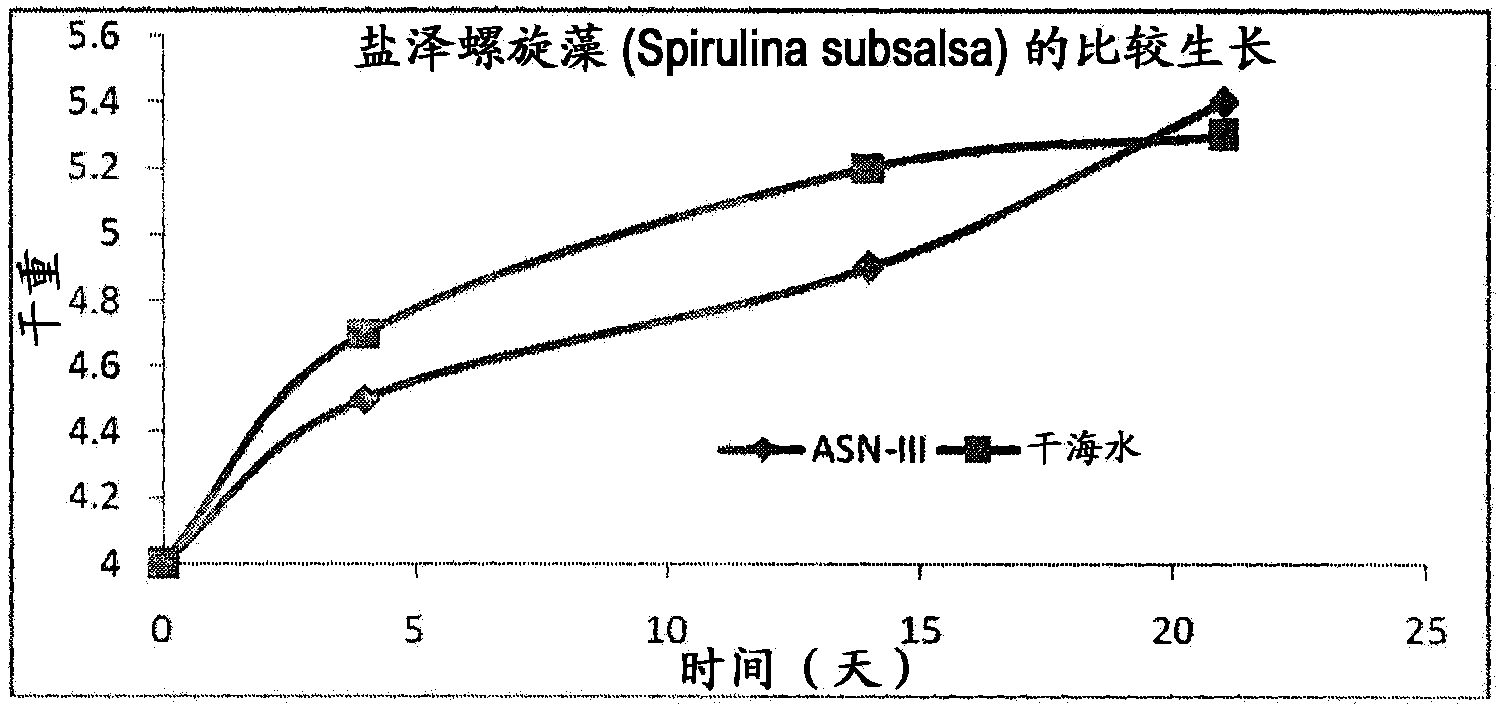
A process for the preparation of natural salt formulations for seawater substitution, mineral fortification
A natural salt and seawater technology, applied in chemical instruments and methods, botanical equipment and methods, food preparation, etc., can solve the problem of expensive products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Composition of 10m3 as Ca 2+ = 0.1% (weight / volume), Mg 2+ =0.27% (weight / volume), Na + = 2.3% (weight / volume), SO 4 2- =0.54% (weight / volume), Cl - =4.1% (weight / volume), K + = 0.08% (w / v) of sea water (7.2°Bé) was transferred to two pots of the same size each having 12m x 2m x 0.23m. These pans are lined with 250 micron LDPE sheet. Seawater was subjected to solar evaporation until 25°Bé.
[0100] a. At 25°Bé, separate 48kg into Ca 2+ = 20.02% (w / w), Mg 2+ = 0.37% (w / w), Na + = 1.1% (w / w), SO 4 2- =52.27% (w / w), Cl - = 2.97% (w / w), K = trace amount of salt as Fraction-I.
[0101] b. The saturated brine at 25°Bé was transferred to another pot of the same size lined with LDPE sheet and subjected to solar evaporation until 28.5°Bé. Make 545kg composed of Ca 2+ = 0.1% (w / w), Mg 2+ = 0.35% (w / w), Na + =38.6% (weight / weight), SO4 2- = 0.62% (w / w), Cl - =59.3% (w / w), K + =Traces of salt crystallized in the second pot and collected as Fraction-II.
[0102]...
Embodiment 2
[0104] Will 5 m3 have Ca 2+ = 0.03% (weight / volume), Mg 2+ = 0.11% (weight / volume), Na + =0.88% (weight / volume), K + =0.03% (weight / volume), SO 4 2- = 0.22% (weight / volume) and Cl - = 1.58% (wt / vol) of seawater with a density of 2.9°Bé was transferred to a solar pot of size 12m x 2m x 0.23m lined with a 250 micron LDPE sheet and allowed to evaporate until 25°Bé.
[0105] a. At 25°Bé, harvest 6kg composed of Ca 2+ = 21.0% (w / w), Mg 2+ = 0.08% (w / w), Na + = 1.1% (w / w), SO 4 2- =52.5% (w / w), Cl - = 2.8% (w / w), K = trace amount of salt as Fraction-I.
[0106] b. Transfer the saturated brine (25°Bé) to another pot of the same size (12m x 2m x 0.23m) lined with a LDPE sheet. Allow this 25°Bé saturated seawater to evaporate until 28.5°Bé and obtain 110kg composed of Ca 2+ = 0.18% (w / w), Mg 2+ = 0.37% (w / w), Na + = 38.6% (w / w), SO 4 2- = 0.62% (w / w), Cl - =59.3% (w / w), K + =Traces of salt as Fraction-II.
[0107] c. The sea brine of 28.5° Bé density remaining after...
Embodiment 3
[0110] Will 5 m3 have Ca 2+ = 0.05% (weight / volume), Mg 2+ = 0.15% (weight / volume), Na + = 1.3% (weight / volume), SO 4 2- =0.3% (weight / volume), Cl - =2.35% (weight / volume), K + = 0.047% (w / v) of seawater with a density of 4.22° Bé was transferred to a pot of dimensions 12m x 2m x 0.23m suitably lined with a 250 micron LDPE sheet.
[0111] a. Allow seawater to evaporate until 25°Bé. Harvest 11kg composed of Ca 2+ = 21.9% (w / w), Mg 2+ = 0.08% (w / w), Na + = 1.1% (w / w), SO 4 2- =51.5% (w / w), Cl - = 2.8% (w / w), K + = Trace amount of salt as Fraction-I.
[0112] b. Transfer the saturated brine (25°Bé) to another pot of the same size (12m x 2m x 0.23m) lined with a LDPE sheet. This 25°Bé saturated seawater was allowed to evaporate until 28.5°Bé. Transfer this brine with a density of 28.5° Bé to the previous pot and obtain 160 kg of composition Ca 2+ = 0.15% (w / w), Mg 2+ = 0.37% (w / w), Na + = 38.6% (w / w), SO 4 2- = 0.62% (w / w), Cl - =59.3% (w / w), K + =Traces of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com