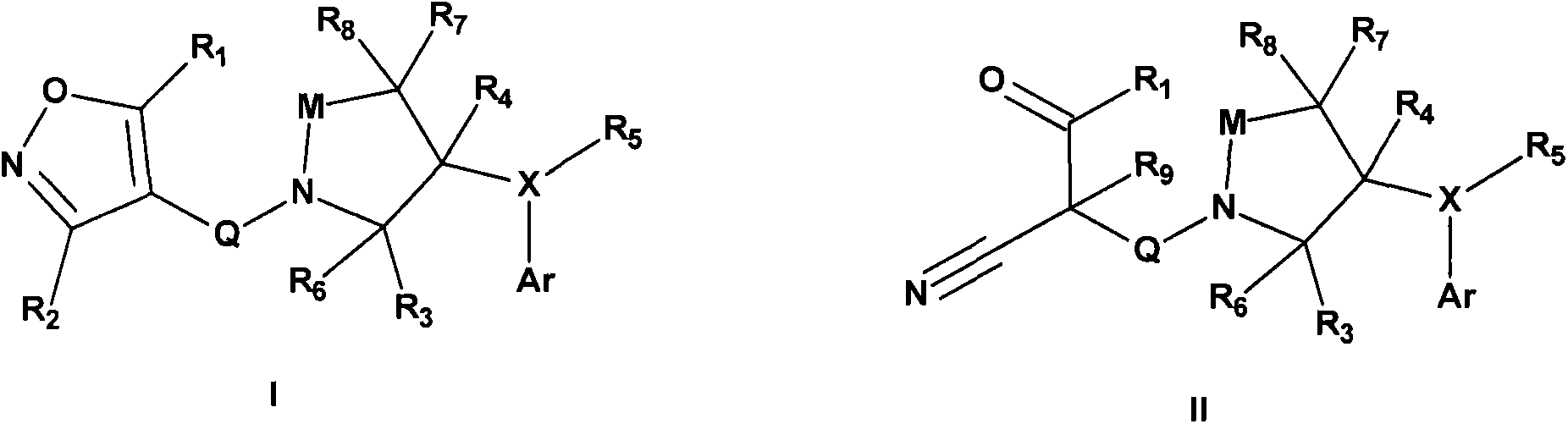
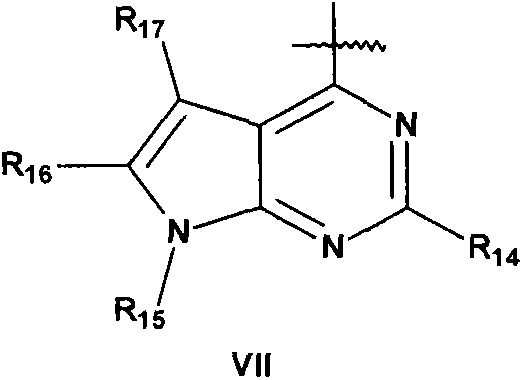
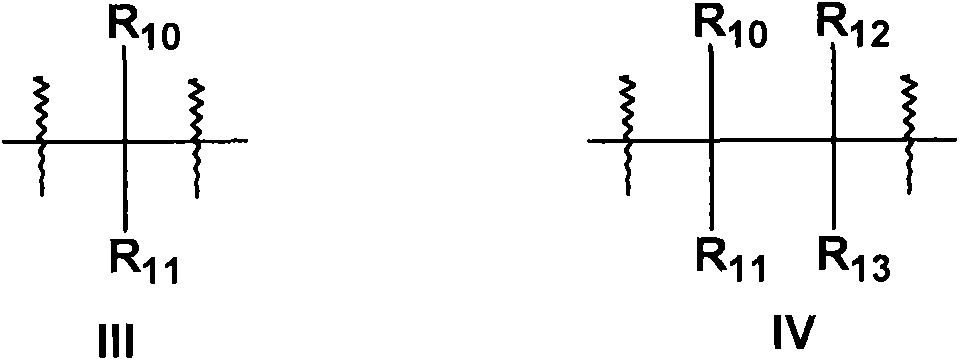New compounds used for prevention and treatment of a plurality of autoimmune diseases
A compound, selected technology, applied in metabolic diseases, skin diseases, bone diseases, etc., can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0077]
[0078] ((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)(5-methyliso Oxazol-4-yl)methanone
[0079] Step A: 4-Chloro-7-(p-toluenesulfonyl)-pyrrolo[2,3-d]pyrimidine
[0080]
[0081] p-Toluenesulfonyl chloride (67.5g, 348mmol, 1.10eq.) and 4-chloro-7-Hpyrrolo[2,3-d]pyrimidine (50g, 320mmol, 1.0eq.) were dissolved in acetone (500ml), At 0°C, sodium hydroxide solution (2Min water, 200ml, 1.20eq.) was slowly added thereto, the reaction was stirred at 20°C for 6 hours, the precipitated solid was filtered and washed with acetone and water to obtain the white target compound (90g , yield=90%).
[0082] LC / MS (method UFLC, total length 2 min): RT = 1.43 min; m / z = 307.9 [M+H] + . 1 H NMR (CDCl 3 400MHz): δ8.80(s, 1H), 8.04-8.15(d, 2H), 7.92(d, 1H), 7.30-7.40(d, 2H), 6.71(s, 1H), 2.39(s, 3H) .
[0083] Step B: N-[(3R,4R)-1-Benzyl-4-methyl-3-piperidine]-N-methyl-7-(p-toluenesulfonyl)pyrrolo[2,3-d] Pyrimidin-4-amine
[0084]
[0...
example 2
[0104]
[0105] 2-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carbonyl)-3-oxo Butyronitrile
[0106] Sodium methoxide (180mg, 3.39mmol, 1.5eq.) dissolved in methanol was slowly added to the compound ((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3- d] pyrimidin-4-yl)amino)piperidin-1-yl)(5-methylisoxazol-4-yl)methanone (0.8g, 2.26mmol, 1.0eq.) in tetrahydrofuran and at 25oC After stirring for two hours, the reaction solution was spin-dried and dissolved in water and adjusted to pH 3 with 2N HCl aqueous solution. The aqueous layer was extracted twice with dichloromethane, the combined organic layer was backwashed once with water, and the organic layer was dried over sodium sulfate. After spin-drying, the target compound (497.8mg, yield=62.5%) was obtained.
[0107] LC / MS (method UFLC, total length 7 minutes): RT=2.45min; m / z=355.2[M+H]+.
[0108] 1H NMR (CD3OD 400MHz): δ8.36(s, 1H), 7.40(d, 1H), 6.94(d, 1H), 5.03(br, 1H), 4.16(d, 2H), 3.97-3.8...
example 3
[0110]
[0111] (3-((7H-pyrrolo[2,3-d]-pyrimidin-4-yl)oxy)-piperidin-1-yl)(methylisoxazol-4-yl)methanone
[0112] Step A: 2-[[4-[(1-Benzyl-3-piperidinyl)oxy]-pyrrolo[2,3-d]-pyrimidin-7-yl]methoxy]ethyl-trimethyl - Silane
[0113]
[0114] Compound 1-benzyl-3-ol (4.82g, 25.2mmol, 1.3eq) was dissolved in 50ml of anhydrous tetrahydrofuran, and NaH (1.55g, 38.8mmol, 2.0eq.60% in mineral oil was added under ice-water bath ). Stirring at room temperature for one hour, 2-[(4-chloro-pyrrolo[2,3-d]-piperidin-7-yl)methoxy]ethyl-trimethyl-silane (5.5g, 19.4mmol, 1.0eq) was dissolved in anhydrous tetrahydrofuran (30mL), then added dropwise to the reaction solution, and stirred overnight at 60oC. Water (30 mL) was added to the reaction solution, followed by extraction three times with ethyl acetate (70 mL). Then it was backwashed once with water and brine, dried over sodium sulfate and spin-dried to obtain the target compound (10 g).
[0115] Step B: 2-[[4-[(1-Benzyl-3-piperidiny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com