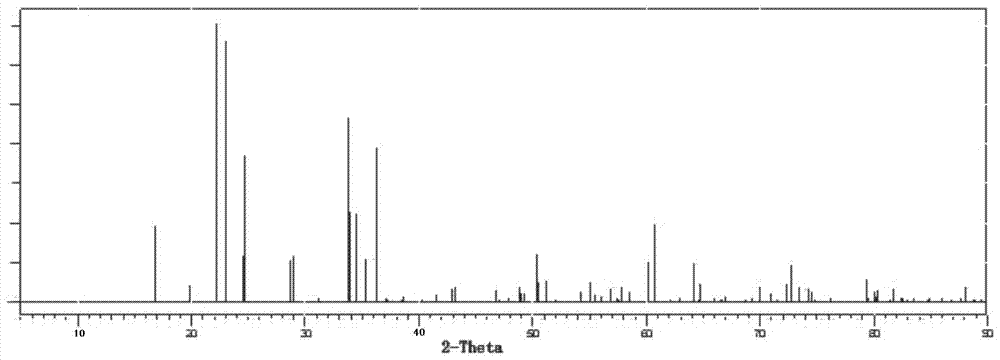
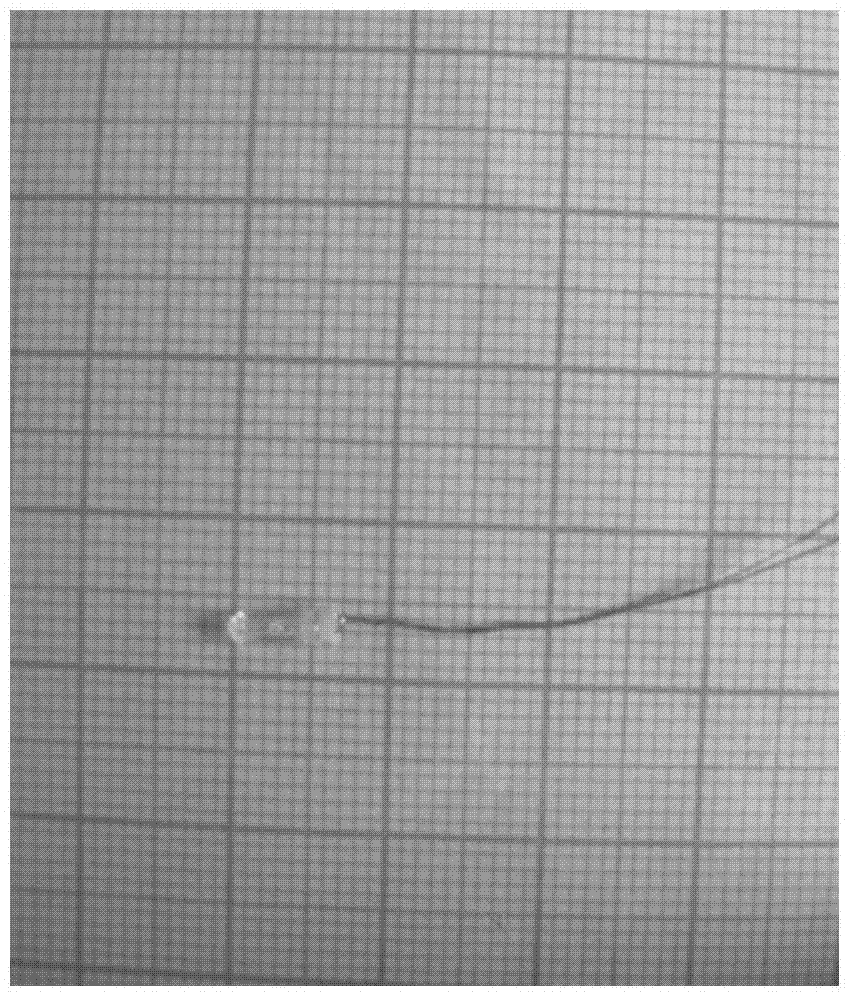
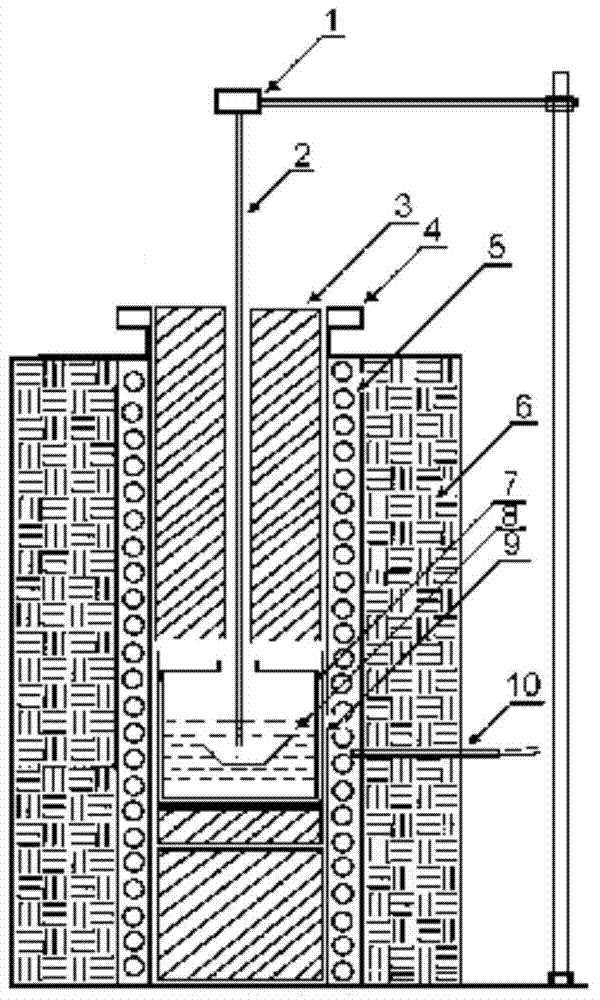
Method for preparing lithium phosphate crystal used as material of lithium battery
A lithium phosphate and crystal technology, applied in the field of optoelectronic materials, can solve the problems of unreported bulk lithium phosphate crystals, and achieve the effects of avoiding internal wrapping and defects, complete crystal form, and high crystal quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Use a combination of LiCl-NaF molar ratio of 1:2 as a flux, raw material lithium carbonate: ammonium dihydrogen phosphate = 3:2 molar ratio and mix evenly into a platinum crucible, control the concentration of solute (ammonium dihydrogen phosphate + lithium carbonate) to 20wt%, heating up to 700°C.
[0032] The chemical reaction equation is: 3Li 2 CO 3 +2NH 4 h 2 PO 4 =2Li 3 PO 4 +2NH 3 ↑+3H 2 O↑+3CO 2 ↑
[0033] Raise the temperature to 800°C and keep the temperature constant for 24 hours to ensure that the material is fully melted and then stirred to make the melt fully mixed and even. Use the seed crystal test method to measure the solution saturation point temperature, select the lithium phosphate seed crystal obtained by spontaneous nucleation without defects, and when the temperature is 4°C higher than the solution saturation point temperature, introduce the seed crystal into the growth furnace, and place it in an appropriate position above the liquid surf...
Embodiment 2
[0035] use MoO 3 -KH 2 PO 4 The molar ratio of 1:3 is used as a flux, the raw material lithium carbonate: ammonium dihydrogen phosphate=3:2 molar ratio is mixed evenly and put into a platinum crucible, and the concentration of the solute (ammonium dihydrogen phosphate + lithium carbonate) is controlled to 20 w t%, heating up to 900°C.
[0036] The chemical reaction equation is: 3Li 2 CO 3 +2NH 4 h 2 PO 4 =2Li 3 PO 4 +2NH 3 ↑+3H 2 O↑+3CO 2 ↑
[0037] Raise the temperature to 900°C and keep the temperature constant for 24 hours to ensure that the material is fully melted and then stirred to make the melt fully mixed and even. Use the seed crystal test method to measure the solution saturation point temperature, select the lithium phosphate seed crystal obtained by spontaneous nucleation without defects, and when the temperature is 4°C higher than the solution saturation point temperature, introduce the seed crystal into the growth furnace, and place it in an appropr...
Embodiment 3
[0039] Use the combination of LiCl-NaF molar ratio 1:1.5 as the flux, the raw material lithium carbonate: ammonium dihydrogen phosphate = 3:2 molar ratio, mix evenly and put it into the platinum crucible, control the concentration of solute (ammonium dihydrogen phosphate + lithium carbonate) to 25wt%, heating up to 750°C.
[0040] The chemical reaction equation is: 3Li 2 CO 3 +2NH 4 h 2 PO 4 =2Li 3 PO 4 +2NH 3 ↑+3H 2 O↑+3CO 2 ↑
[0041] Keep the temperature at 750°C for 26 hours, and stir evenly after the material is fully melted. Seed according to the method of Example 1, then lower the temperature to 1°C above the saturation point, rotate at a rotation rate of 35 revolutions per minute, rotate in a forward-stop-reverse cycle, and start cooling after 4.5 hours. The cooling rate is 0.2-0.6°C / day for the first 16 days in the early growth period, 0.5-1.5°C / day for the second 16 days in the mid-growth period, and 1-2°C / day for the third 16 days in the later stage. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com