Compounds for use in the treatment of feline retroviral infections
A technology of compounds and solvates, which can be used in the fields of compounds, antiviral agents, and drug combinations of elements of Group 5/15 of the periodic table, which can solve problems such as inability to avoid infection and inability to induce sterile immunity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: Inhibition of Feline Leukemia Virus Replication by R-PMPDAP in Cell Culture
[0134] Materials and Methods
[0135] The compound was dissolved in 0.05N sodium hydroxide solution at a concentration of 10 mg / ml. Crandell Reese feline kidney (CrFK) cells were grown in Dulbecco's minimal essential medium (DMEM, Life Technologies) supplemented with 1% sodium bicarbonate (Life Technologies) and 5% fetal calf serum (FCS, Biochrom). In a 96-well plate, seed 5000 CRFK cells and store at 37°C with 5% CO 2 Incubate for 24 h in a humid atmosphere. Cells were subsequently washed with 100 μl of phosphate-buffered saline (PBS) containing 50 μg / ml DEAE-dextran and seeded at 100 CCID in the presence of increasing doses of compound (0.4, 2, 10 and 50 μg / ml) 50 FeLV. After incubation for 2 h to allow the virus to enter the cells, the supernatant was removed and the cells were washed with PBS. DMEM containing 5% FCS with increasing doses of compound was added to infected ...
Embodiment 2
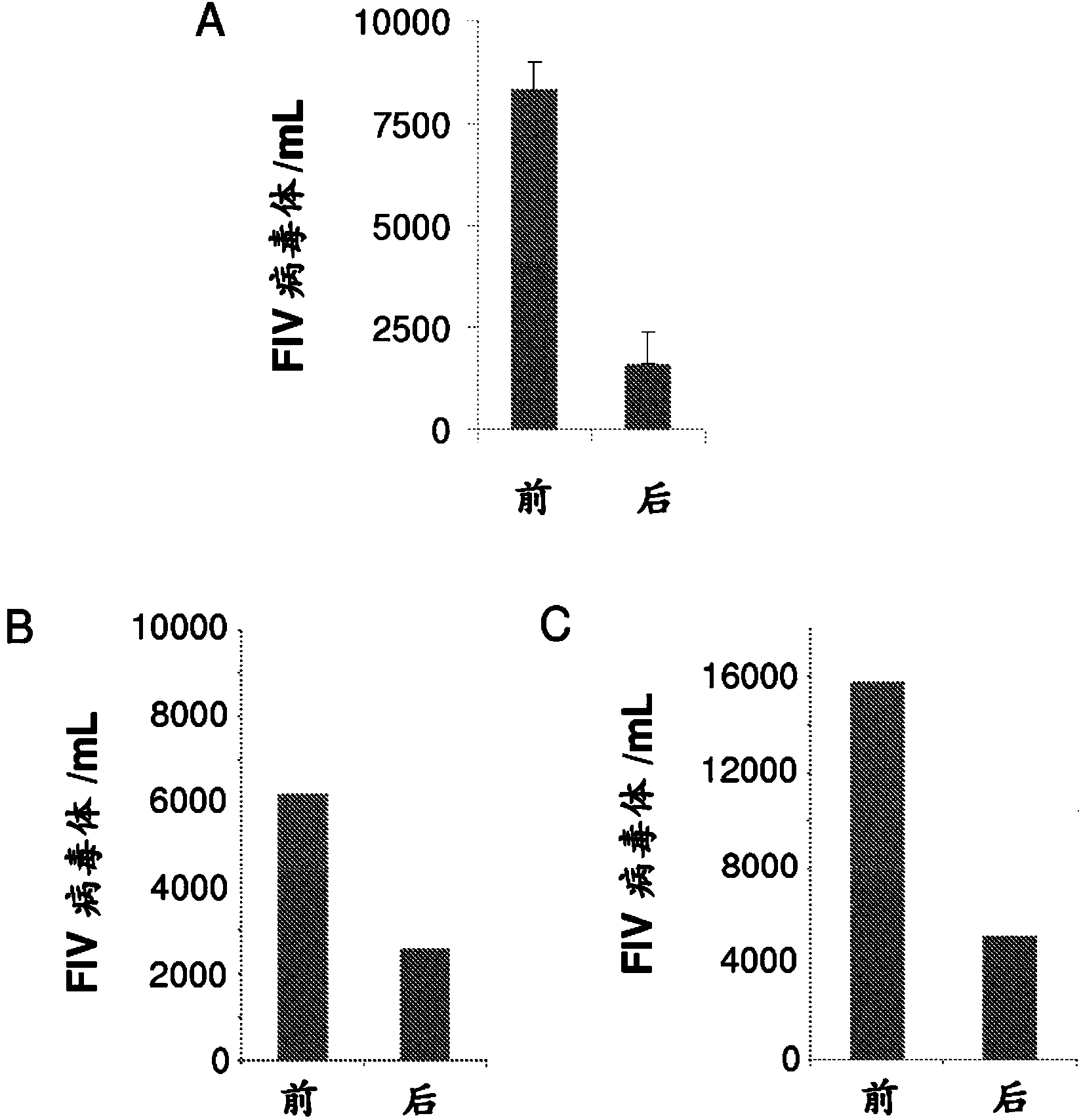
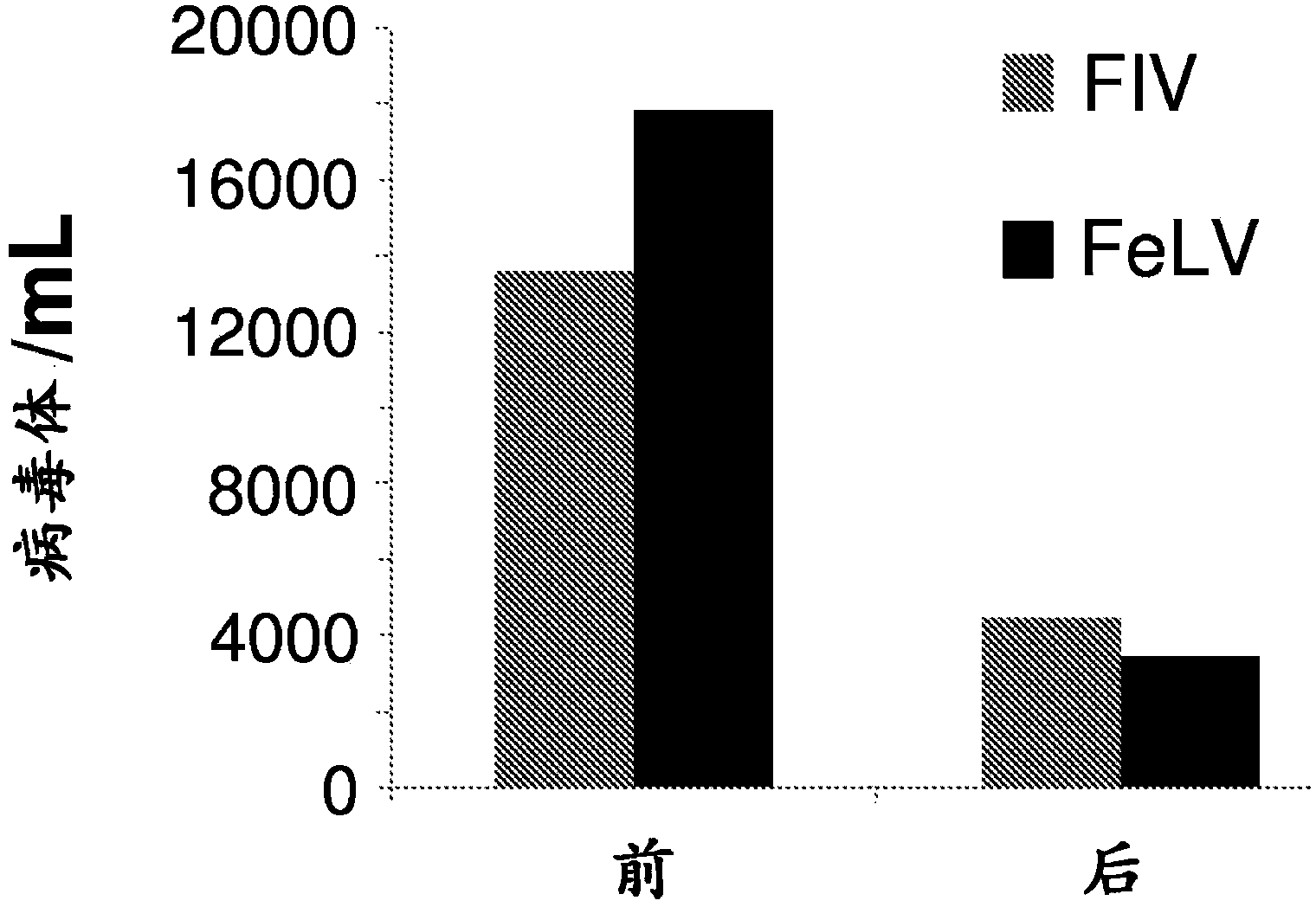
[0140] Embodiment 2: R-PMPDAP treatment to the clinical disease cat of natural infection FIV
[0141] Materials and Methods
[0142] Compounds are dissolved in a suitable aqueous buffered sodium hydroxide solution at near neutral pH conditions (pH 6-9) to a concentration of 50-250 mg / mL.
[0143] The formulated compound is administered by the subcutaneous route at least once a week at a total dosage rate of 10-175 mg / kg per week. The duration of treatment is from 1 week to 3 months. Treated cats were clinically diseased cats naturally infected with FIV and exhibited various symptoms.
[0144] result
[0145] Treatment outcomes for cats with specific symptoms are discussed below.
[0146] • Cats with oral signs.
[0147] Oral signs may manifest themselves, but are not limited to, stomatitis, gingivitis, laryngitis, glossitis, periodontitis, gingivostomatitis, tonsil and tongue ulcers, salivation, and the like. Examples of the effect of said compounds in the treatment ...
Embodiment 3
[0170] Example 3: R-PMPDAP Treatment of Cats Naturally Infected with FeLV
[0171] Materials and Methods
[0172] The compound is dissolved in an appropriate aqueous buffered sodium hydroxide solution at a concentration of 50-250 mg / ml and near neutral pH conditions (pH 6-9). The formulated compound is administered by the subcutaneous route at least once a week at a total dosage rate of 10-175 mg / kg per week. The duration of treatment is from 1 week to 3 months.
[0173] result
[0174] ●Cats with high viral loads (viremic cats)
[0175] FeLV-positive female cats whose FeLV viral load was quantified using RT-qPCR (Cattori and Hofmann-Lehmann, 2008) were treated with the compounds. Viral load decreased significantly from 15500 FeLV virions / mL plasma to 3200 FeLV virions / mL plasma.
[0176] ●Clinically symptomatic cats
[0177] FeLV-positive male cats with FeLV-related clinical signs such as stomatitis, lymphadenopathy, diarrhea, etc. resulting in a low Karnofsky score...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com