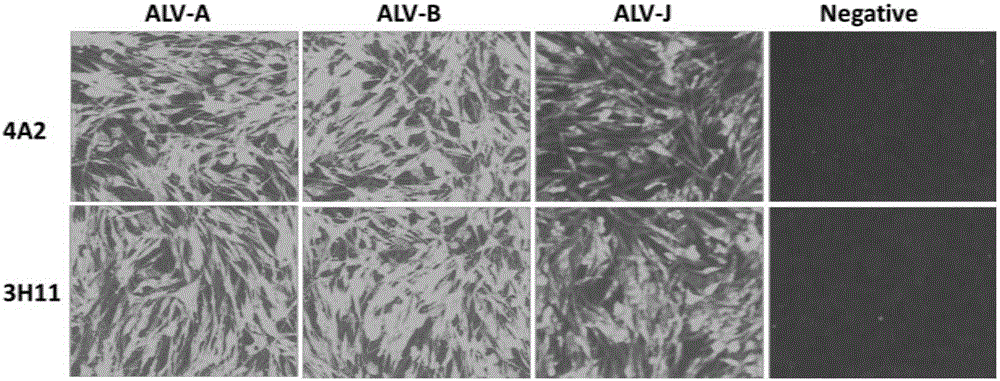
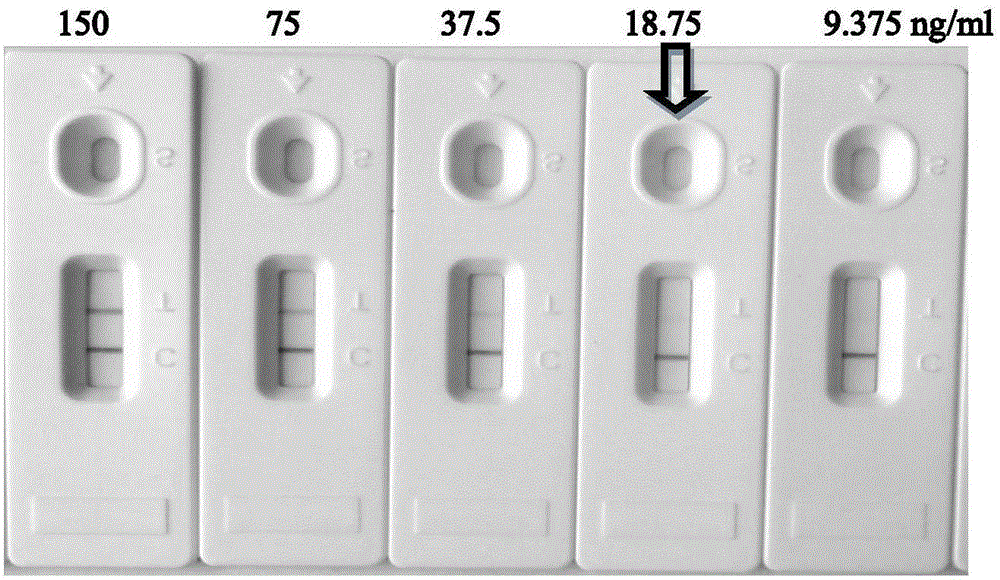
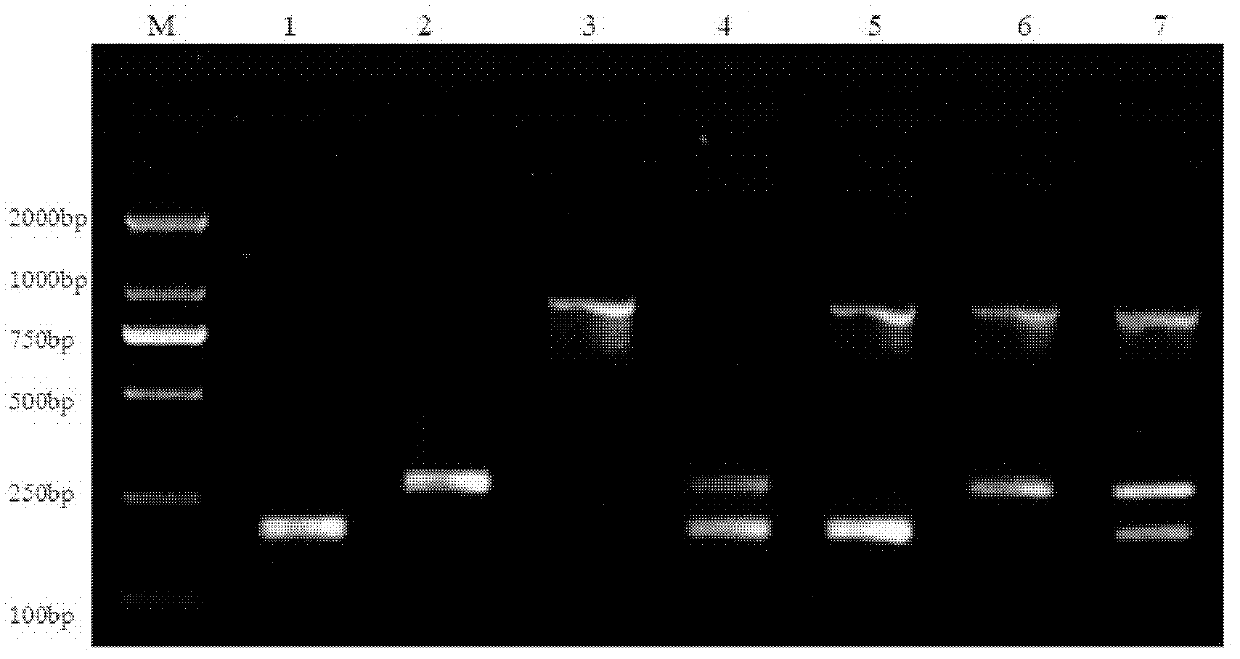
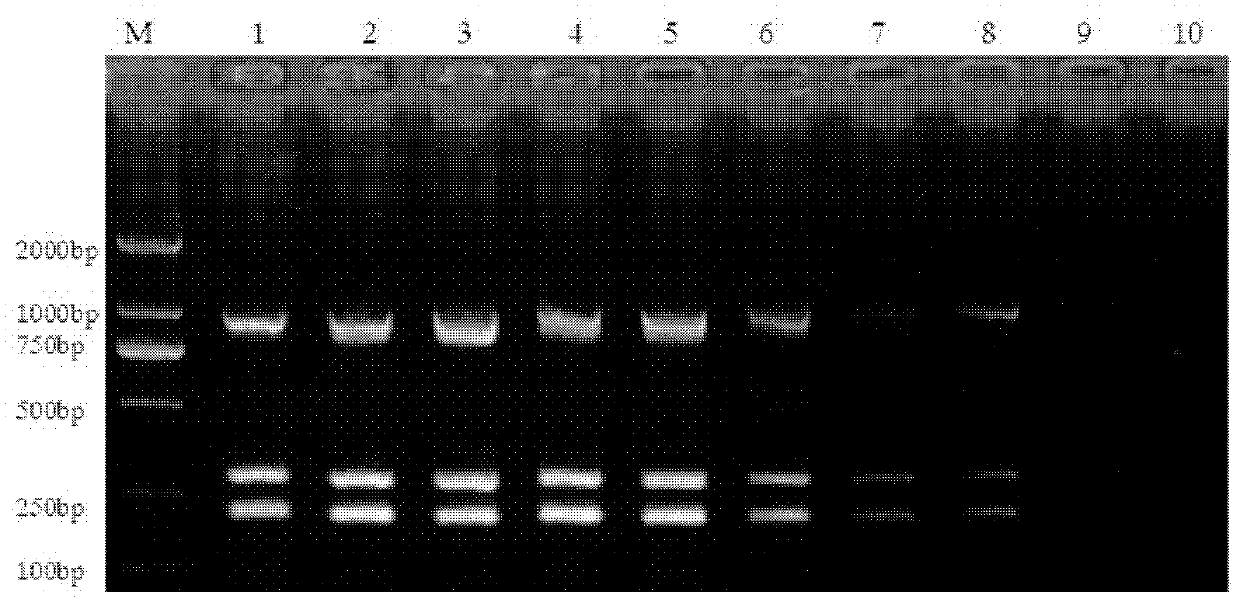
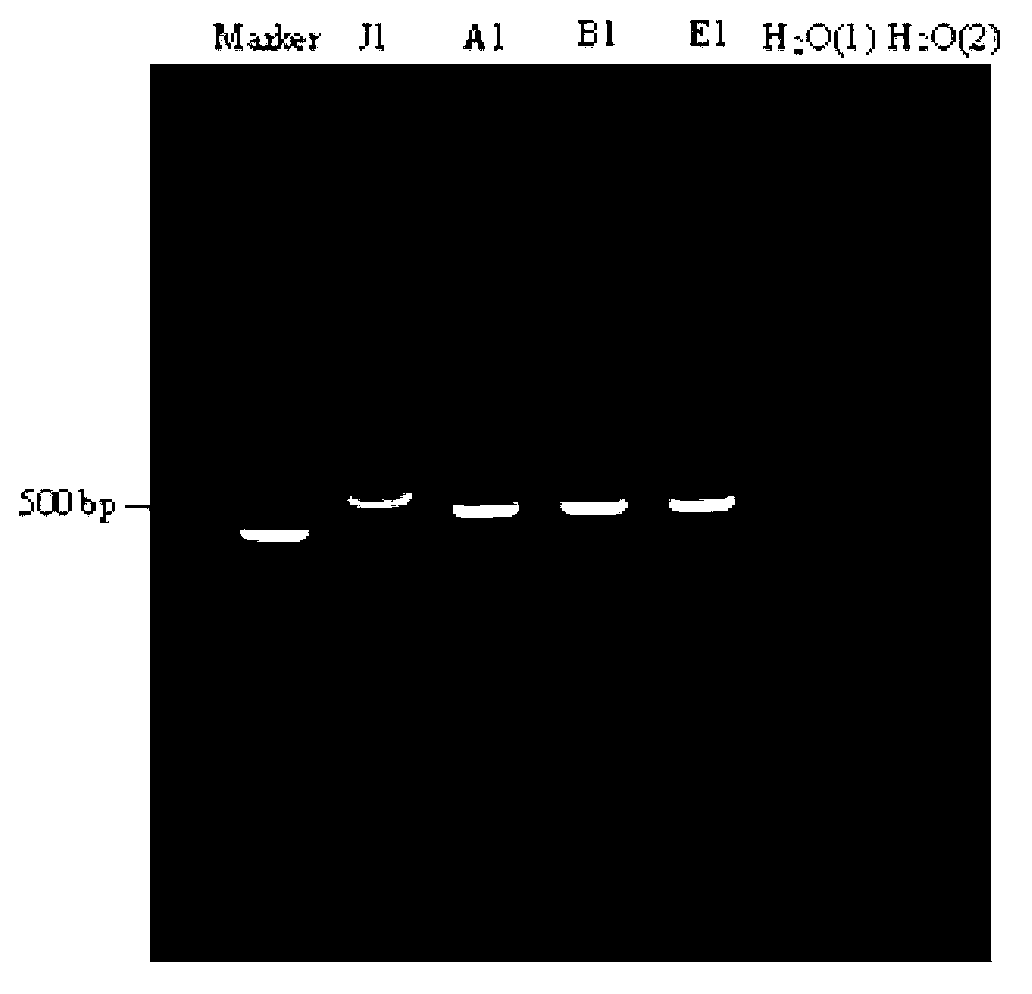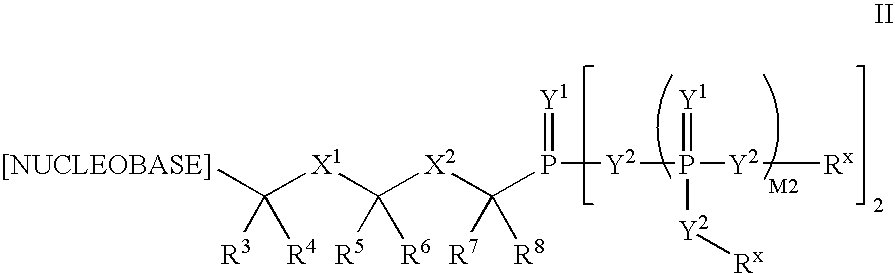Patents
Literature
119 results about "Leukemogenic Viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
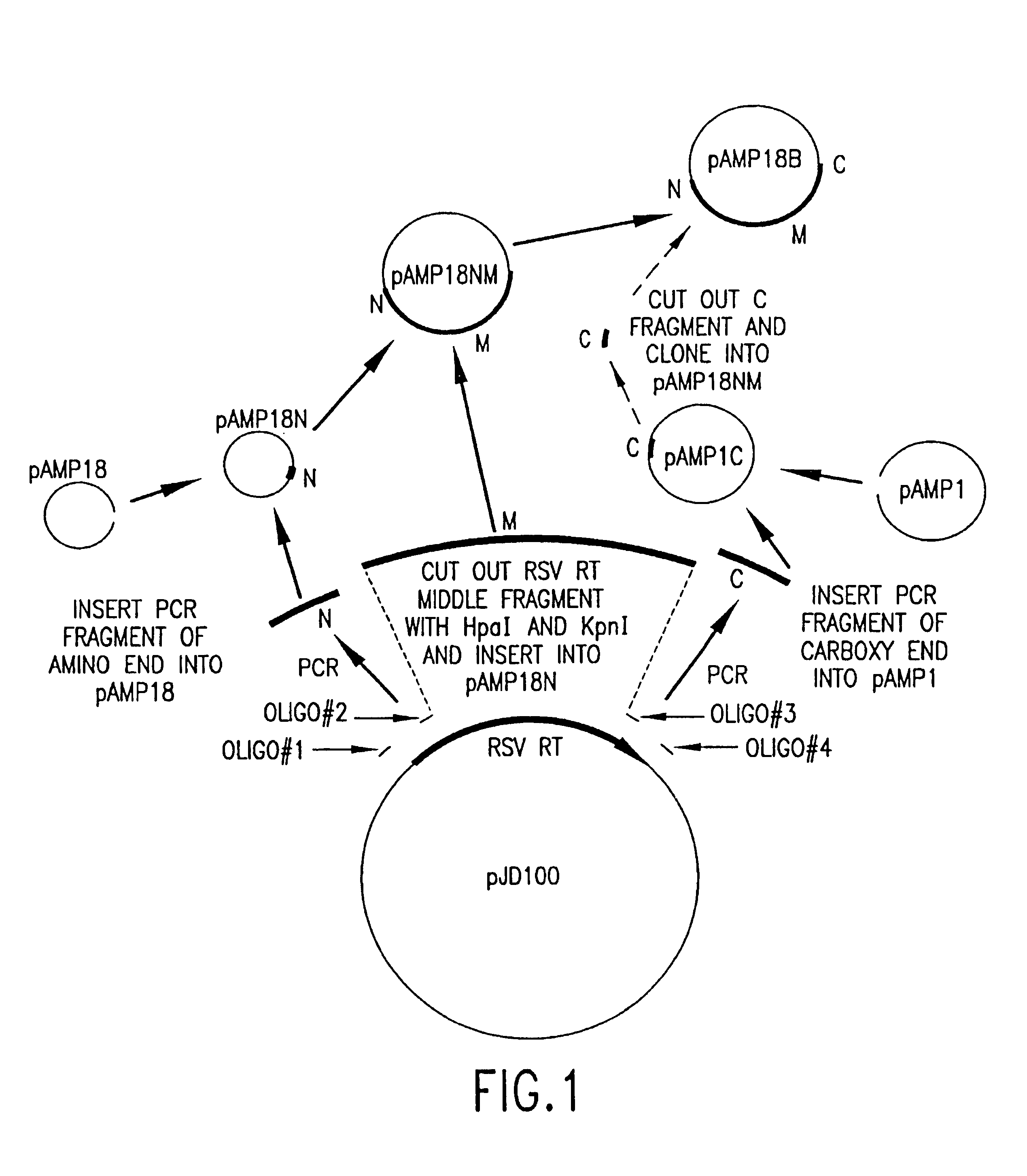
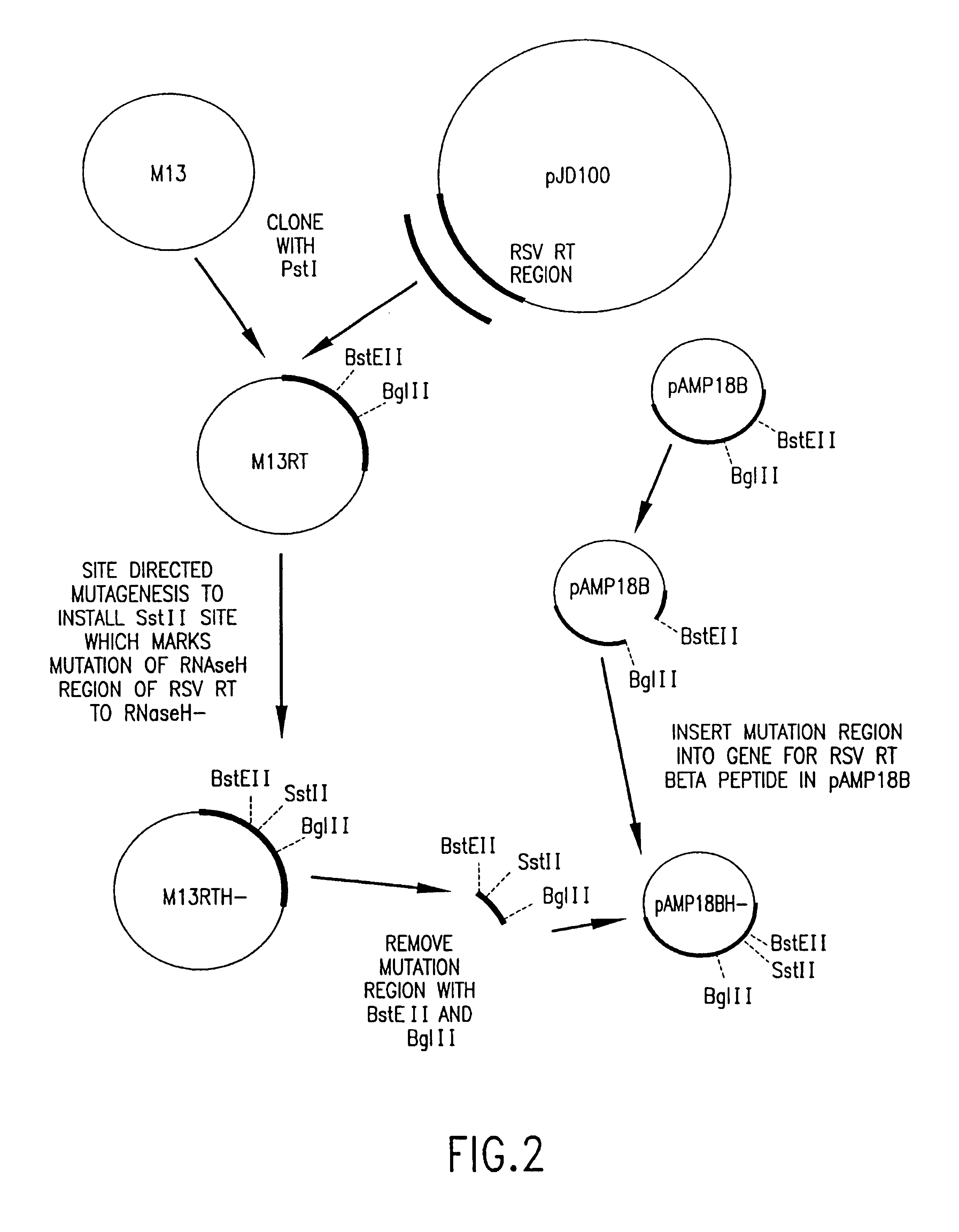
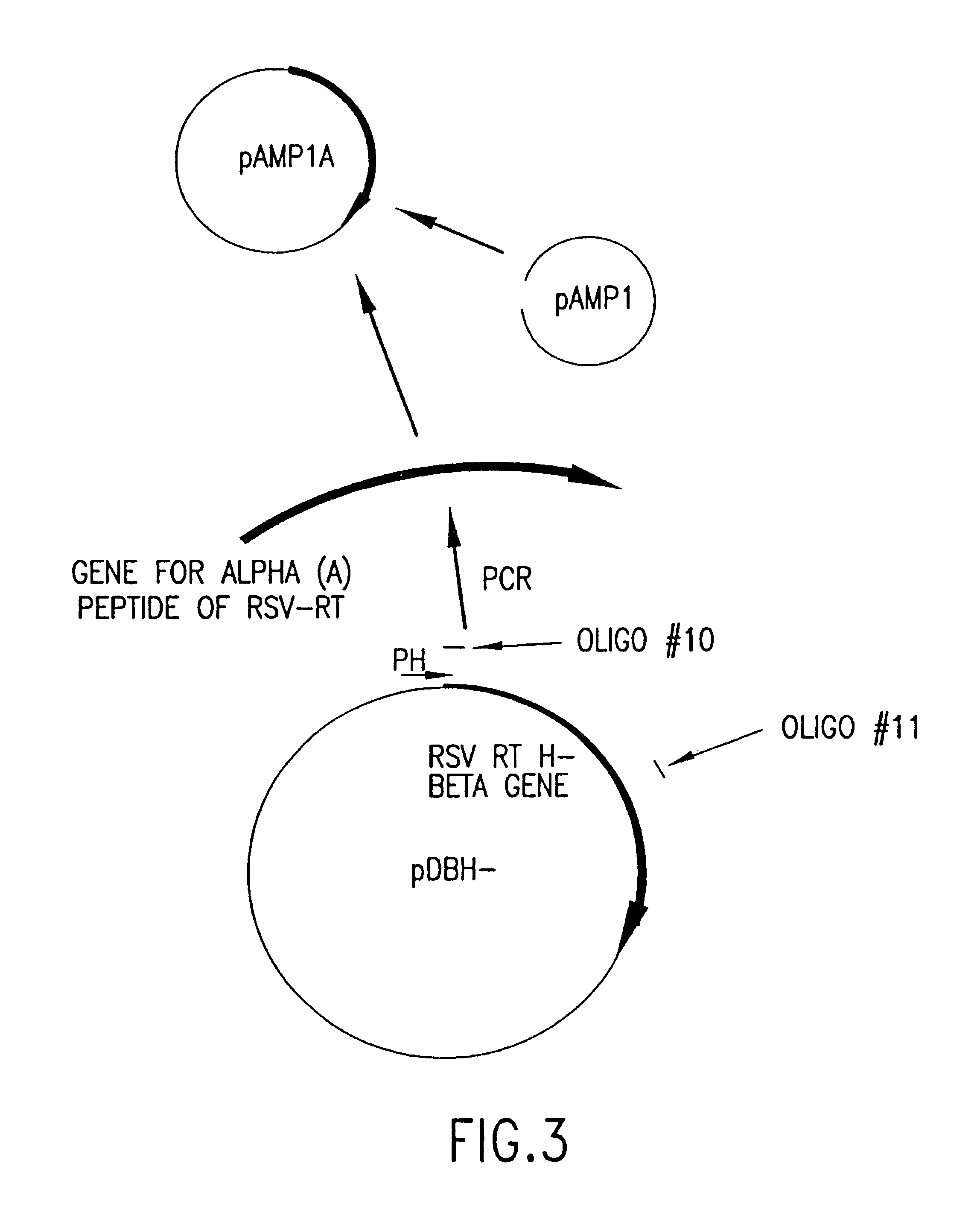
Recombinant methods for making reverse transcriptases and mutants thereof
InactiveUS6989259B2Reduced and substantially reduced in RNase H activityFungiBacteriaAvian leukosis virusesAvian sarcoma leukosis virus
The invention relates to compositions comprising mixtures of polypeptides having reverse transcriptase (RT) activity and to methods of producing, amplifying or sequencing nucleic acid molecules using these compositions or polypeptides, particularly at temperatures above about 55° C. The invention also relates to nucleic acid molecules produced by these methods, to vectors and host cells comprising these nucleic acid molecules, and use of such nucleic acid molecules to produce desired polypeptide. The invention also relates to methods for producing Avian Sarcoma-Leukosis Virus (ASLV) RT subunits, in particular, Avian Myeloblastosis Virus (AMV) RTs, to isolated nucleic acid molecules encoding ASLV RT subunits, and to ASLV RT subunits produced by these methods. The invention further relates to nucleic acid molecules encoding recombinant RT holoenzymes, particularly ASLV RTs, methods for producing these RTs and to RTs produced by these methods. The invention also relates to kits comprising the compositions, polypeptides, and ASLV RTs of the invention.
Owner:LIFE TECH CORP
Anti-avian leukosis virus p27 protein monoclonal antibody, gold-colloidal strip containing same and application
ActiveCN105198986AHigh potencyStrong specificityMicroorganism based processesImmunoglobulins against virusesProtein.monoclonalAvian leukosis viruses
The invention discloses an anti-avian leukosis virus p27 protein monoclonal antibody, a gold-colloidal strip containing the same, and application. The anti-avian leukosis virus p27 protein monoclonal antibody is generated by secretion of a hybridoma cell strain with a preservation number of CGMCC NO. 11089 or a hybridoma cell strain with a preservation number of CGMCC NO. 11090. The monoclonal antibody is high in titer to p27 protein and good in specificity, and therefore can be used for preparing a kit and the gold-colloidal strip for detecting an avian leukosis virus. A preparation method of the monoclonal antibody provided by the invention is simple, and an antibody purification process is simple, and the efficiency is high, and the cost is low. As the gold-colloidal strip prepared by the anti-avian leukosis virus p27 protein monoclonal antibody provided by the invention is adopted to detect the anti-avian leukosis virus, the specificity is strong, and the operation is simple, and convenience, speediness and simplicity are realized, besides, a special instrument and equipment are not needed, and professional training is not needed, and a result is clear and easy to recognize; the operation is simple and convenient; the popularization is easy, and the antibody is more suitable for real-time detection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multiplex PCR (polymerase chain reaction) detection primers for avian leukosis viruses and application thereof
InactiveCN102433397ALow costImprove quality requirementsMicrobiological testing/measurementDNA/RNA fragmentationMultiplexLeucosis
The invention discloses multiplex PCR (polymerase chain reaction) detection primers for avian leukosis viruses. The primers comprise detection primers for detecting subgroup A, subgroup B and subgroup J of avian leukosis viruses. The inventional also discloses a reaction system and reaction conditions of multiplex PCR. The detection method provided by the invention has the advantages of being rapid, accurate and cheap.
Owner:GANSU AGRI UNIV
Multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting five cow disease viruses
ActiveCN105002301AGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesLeucosisBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Retroviral Vector Particles and Methods for their Generation and Use
InactiveUS20120258494A1Low costIncreased susceptibilityVectorsVertebrate cellsTherapeutic proteinMurine leukaemia virus
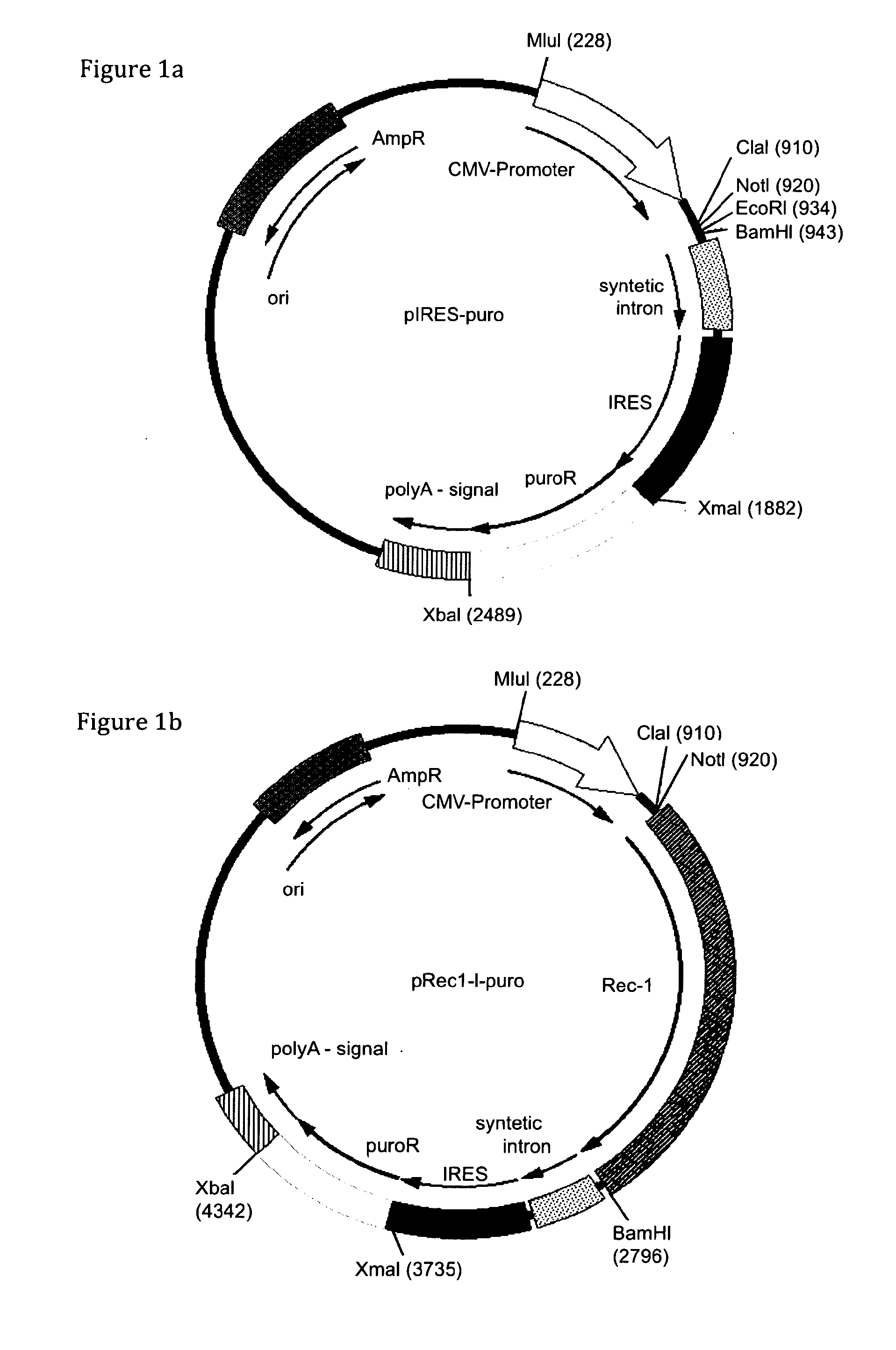
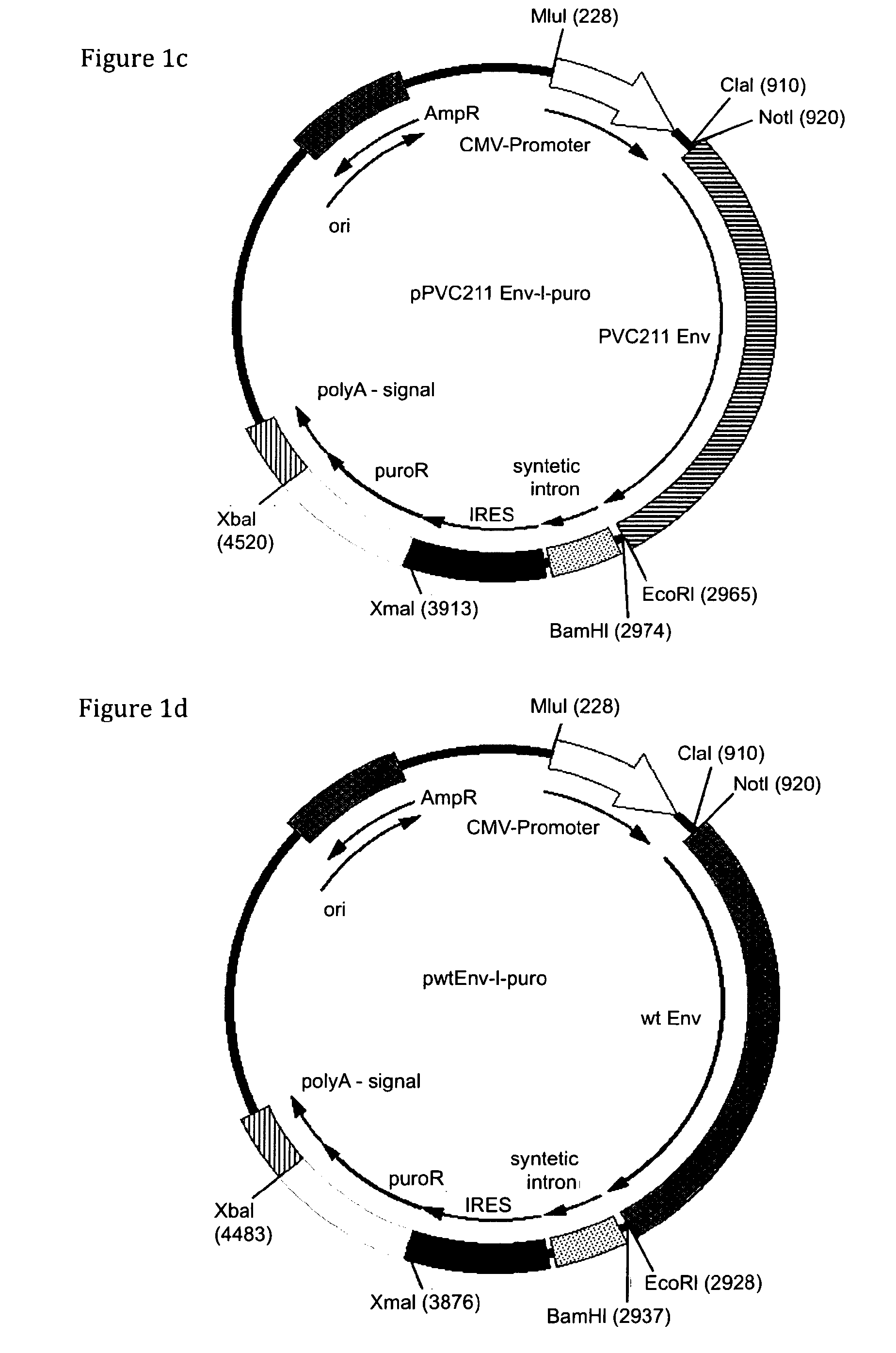
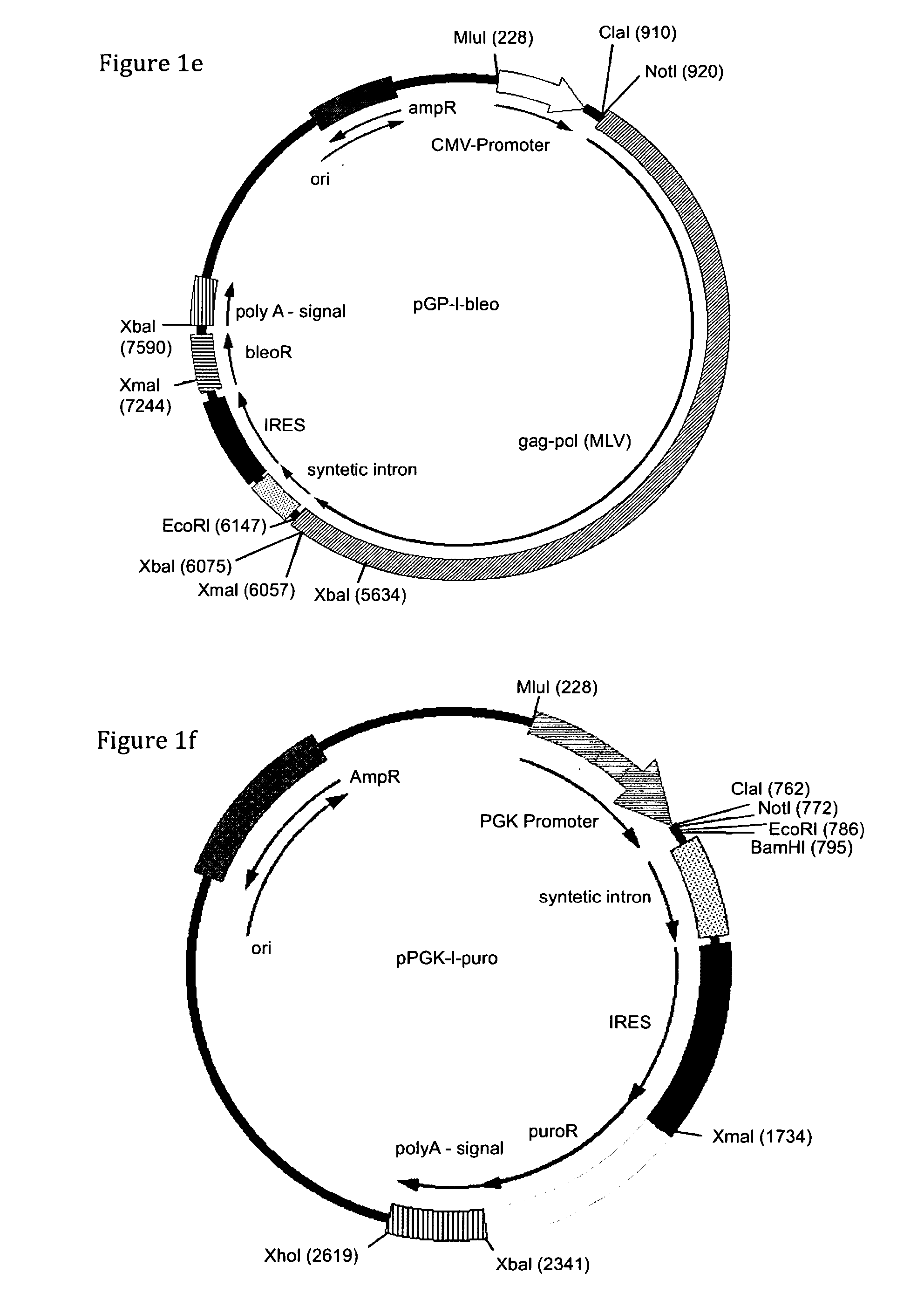
The present invention relates to methods of host cell transduction utilising ecotropic retroviral vector particles. The retroviral vector particle may comprise an envelope of Friend murine leukaemia virus, in particular the envelope encoded by molecular clone PVC-211 and the host cell may be engineered to recombinantly express the Reel receptor. The retroviral vector particles and methods of the invention can be used to introduce expressible polynucleotide sequences of interest into host cells with high efficiency. This results in protein production methods with higher yield (mg / L) and a reduction in manufacturing costs that could be used in a range of applications including for example, the production of therapeutic proteins, vaccines and antibodies.
Owner:AGENUS INC
Moloney murine leukemia virus reverse transcriptase mutant as well as expression method and application thereof
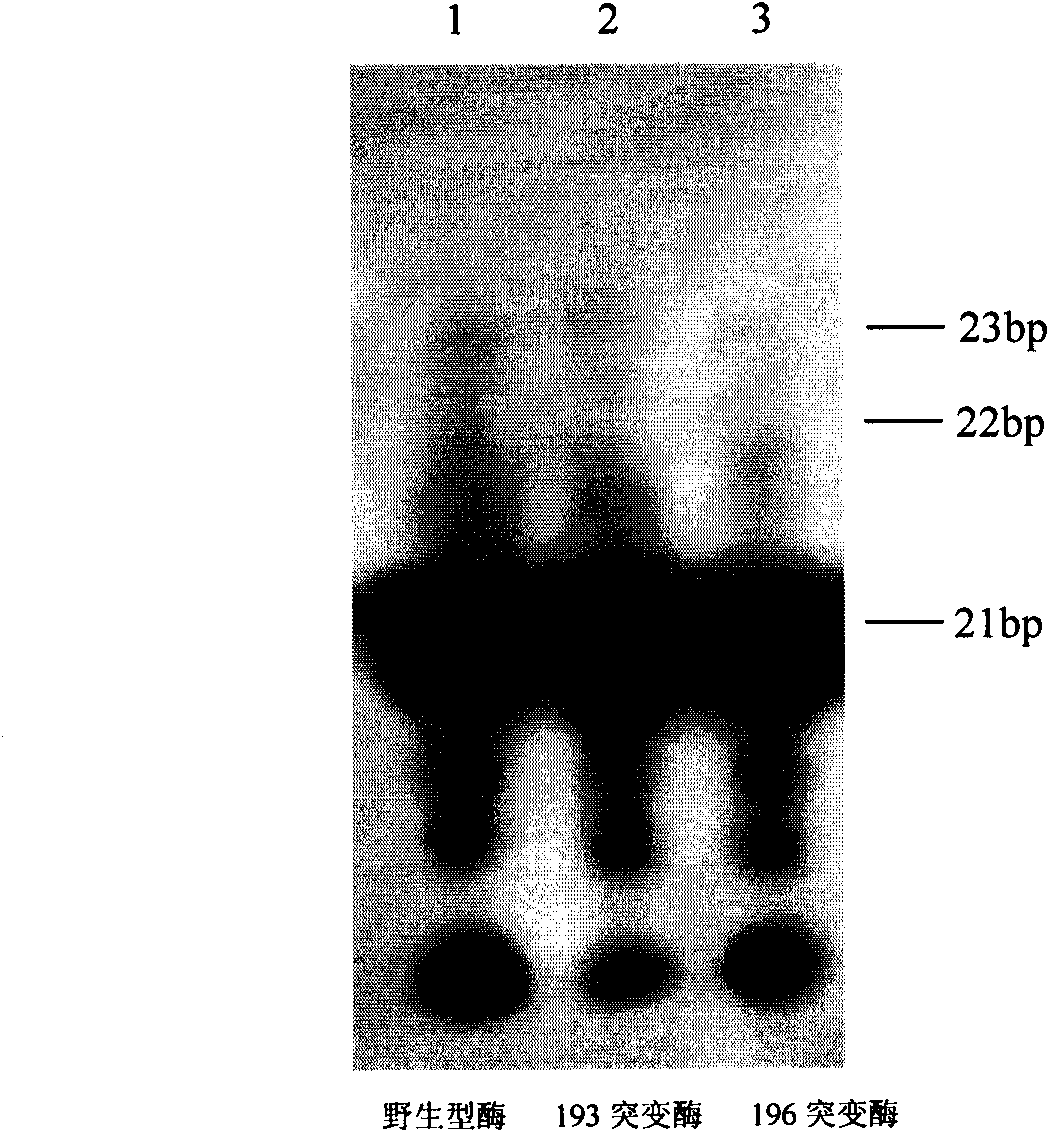
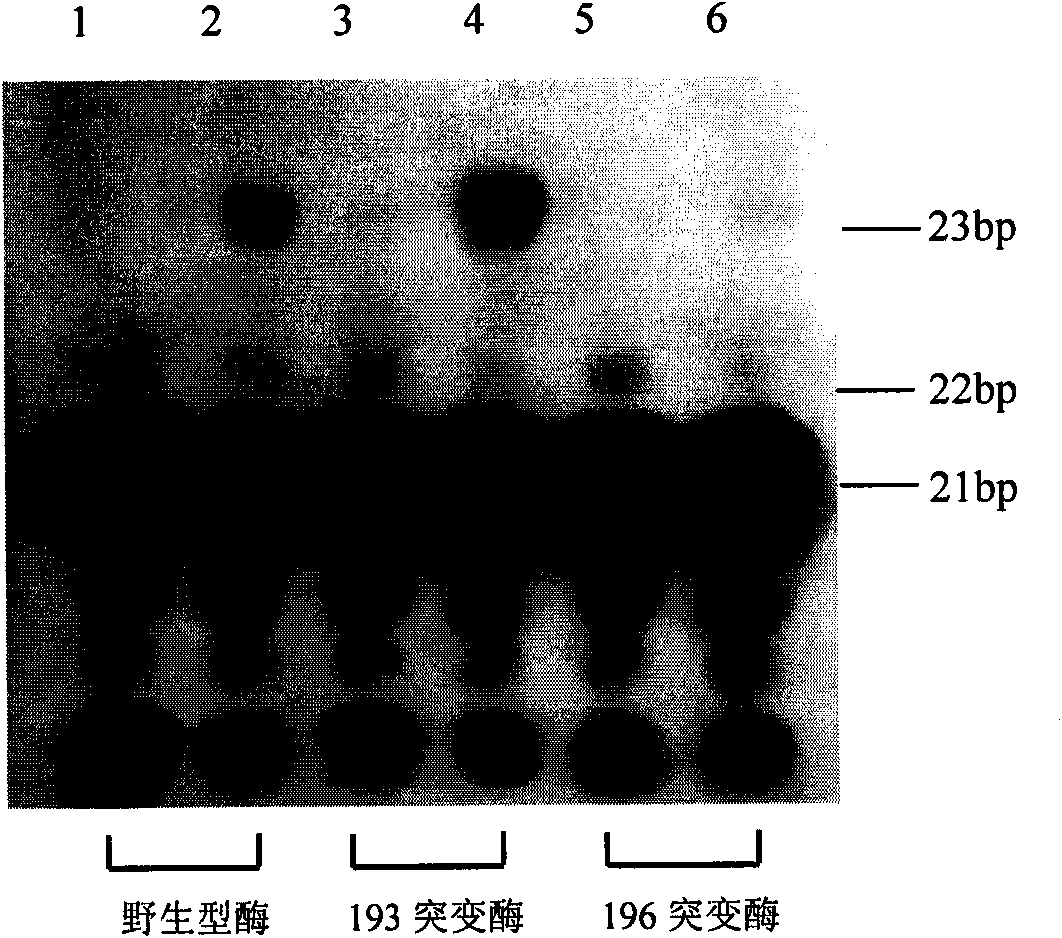
The invention provides a moloney murine leukemia virus reverse transcriptase mutant as well as an expression method and application thereof. The mutant is a protein formed by substituting a praline residue at the 196th site of the moloney murine leukemia virus reverse transcriptase from the N end by an alanine residue. The expression method of the moloney murine leukemia virus reverse transcriptase comprises the steps of transforming an expression vector containing the coding gene of the moloney murine leukemia virus reverse transcriptase into Escherichia coli, culturing positive clones and expressing to obtain the moloney murine leukemia virus reverse transcriptase mutant. The mutant can be applied to RNA synthesis, and the reverse transcriptase mutant obtained from reconstructing the moloney murine leukemia virus reverse transcriptase has a fidelity function.
Owner:GUANGZHOU HUAYIN MEDICINE SCI & TECH +1
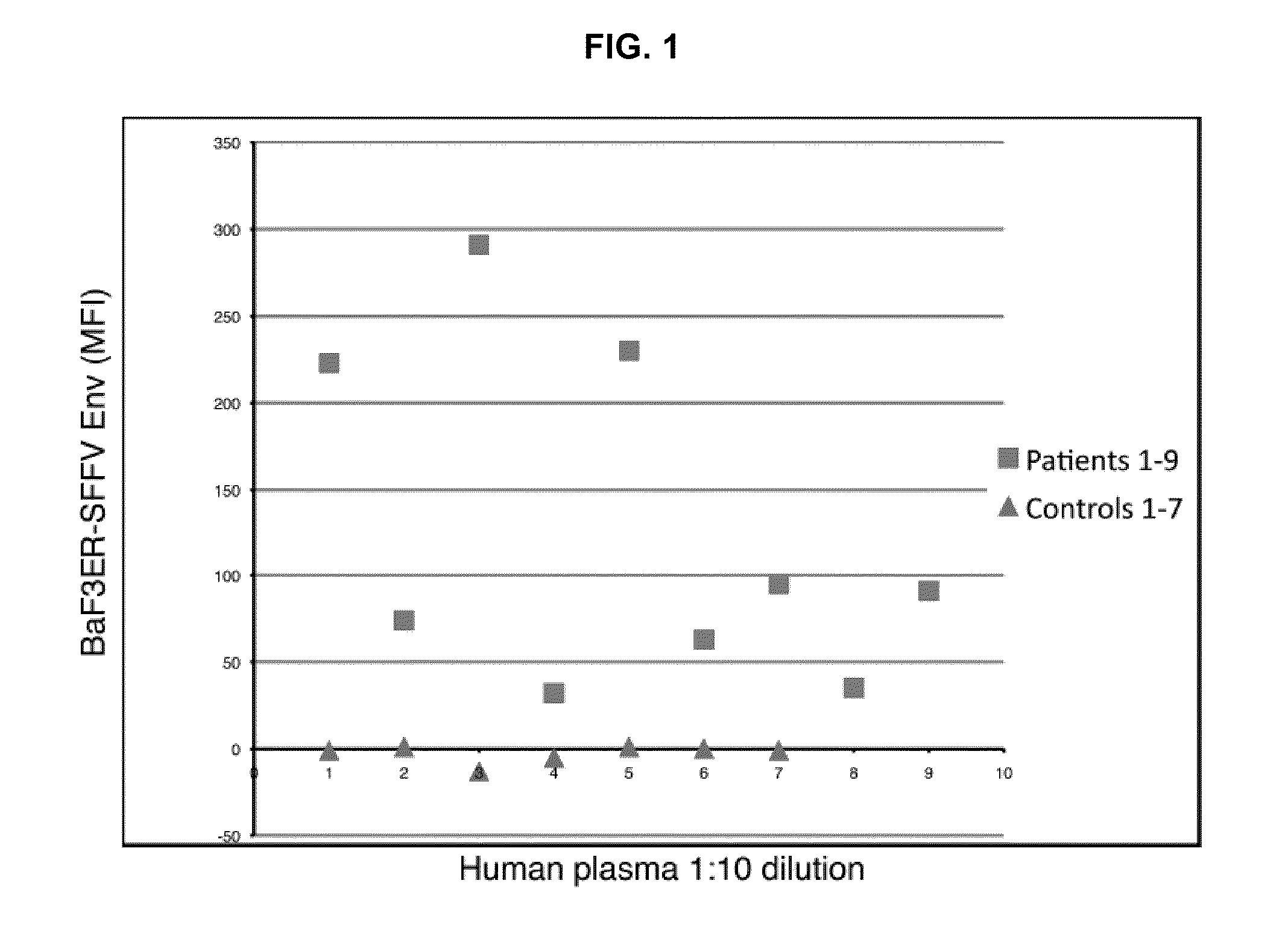
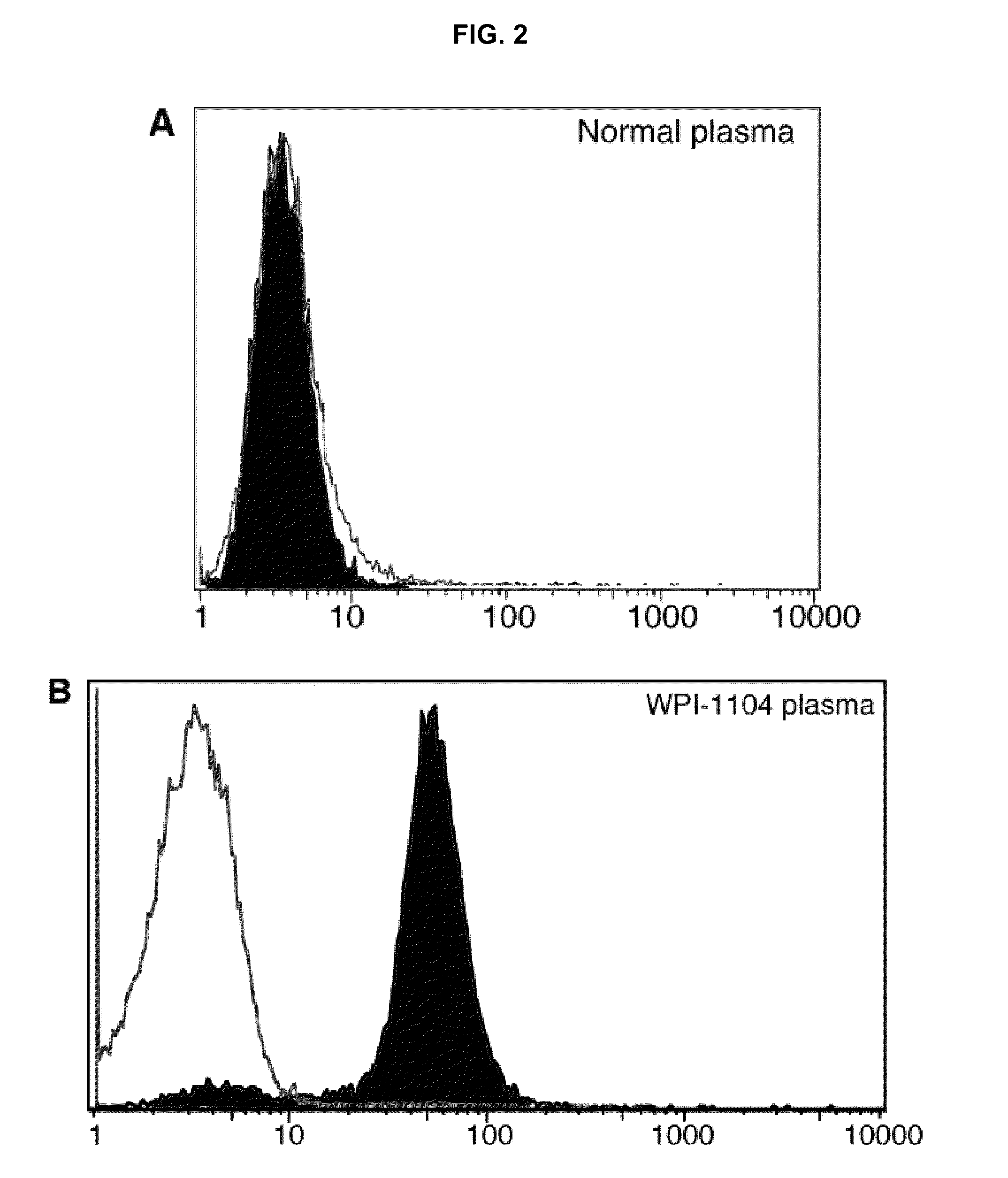
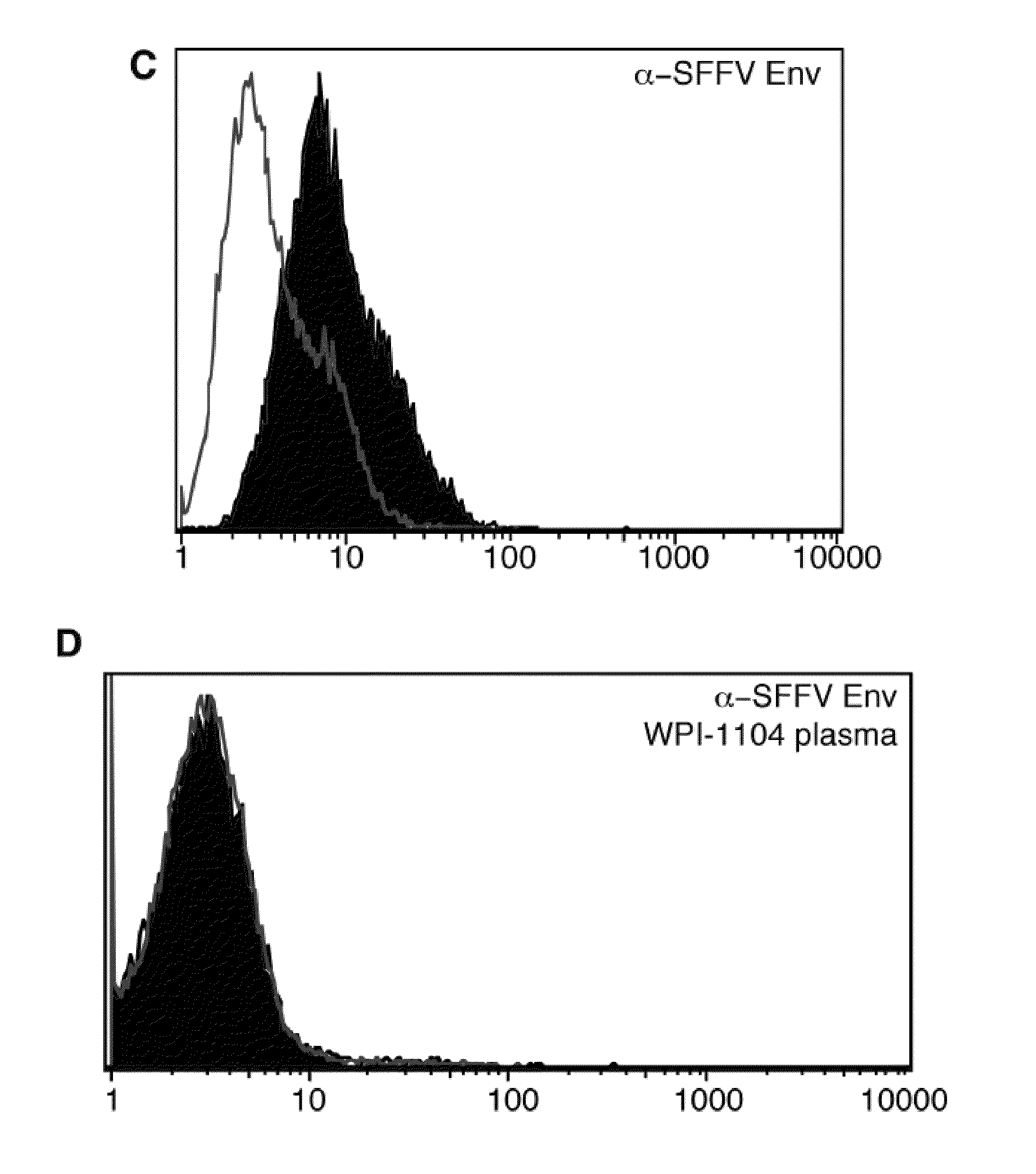
Seroconversion assays for detecting xenotropic murine leukemia virus-related virus
Methods of detecting, diagnosing, monitoring or managing an XMRV-related disease such as an XMRV-related neuroimmune disease such as chronic fatigue syndrome or an XMRV-related lymphoma such as mantle cell lymphoma in a subject are disclosed. These methods comprise determining presence, absence or quantity of antibodies against XMRV in a sample from a subject.
Owner:WHITTEMORE PETERSON INST FOR NEURO IMMUNE DISEASE
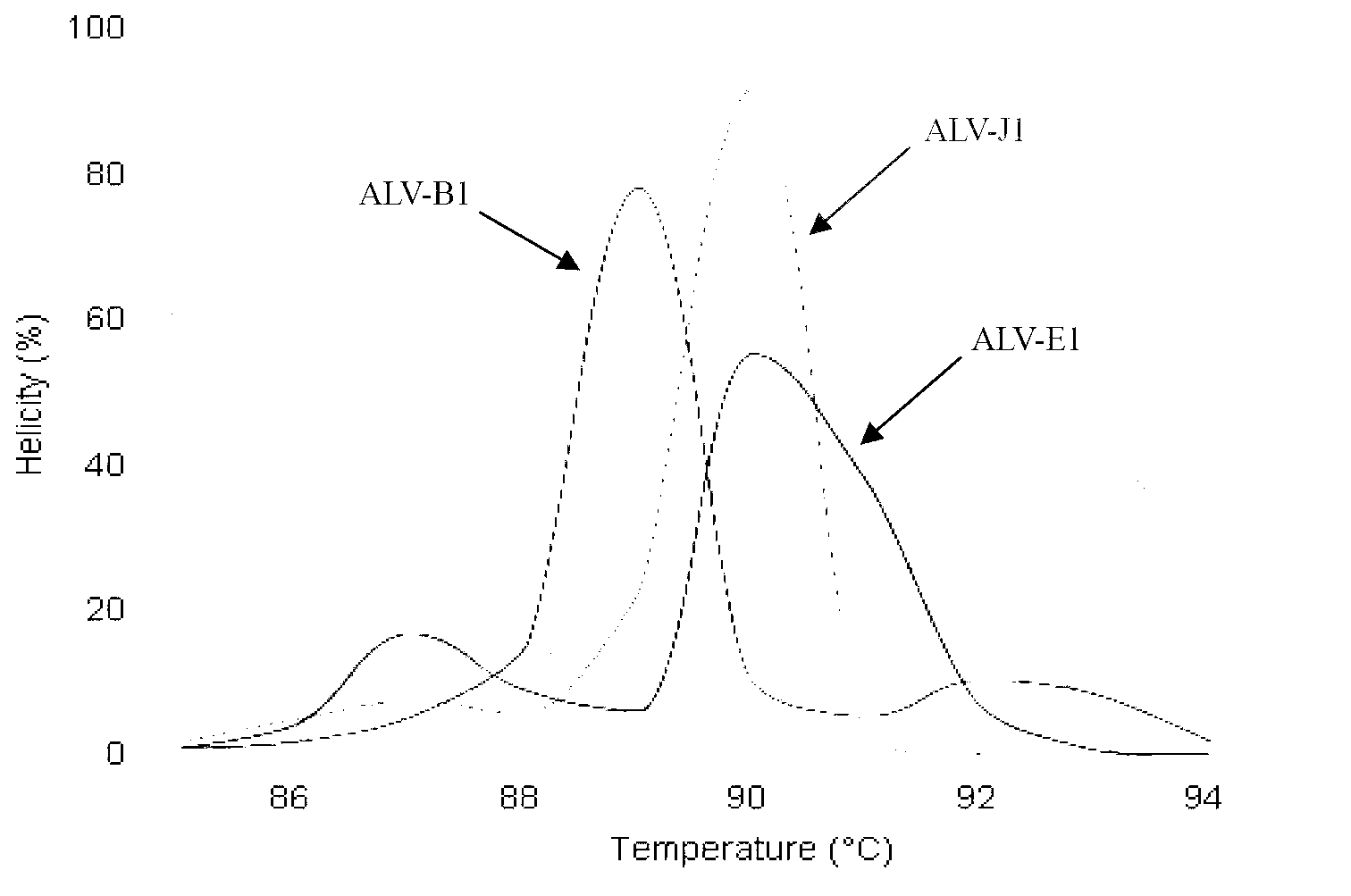
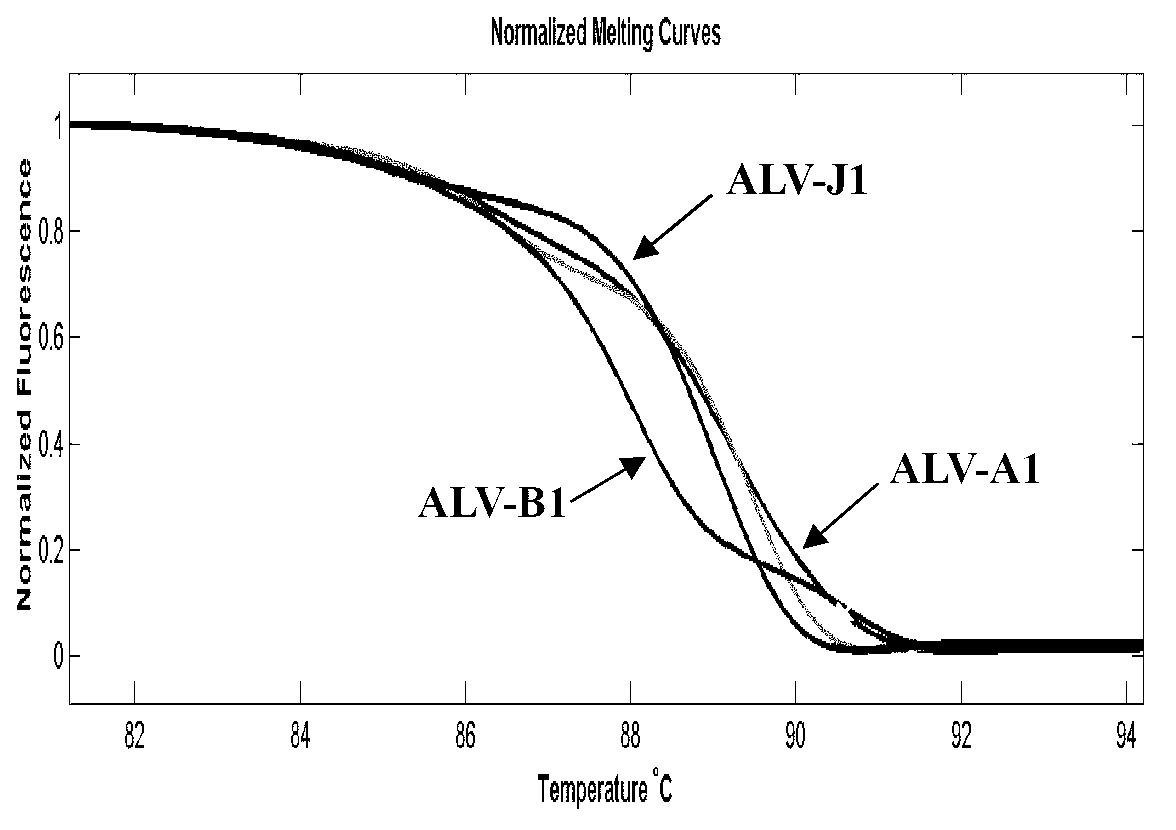
Establishment of PCR-HRM analysis method for rapid differential diagnosis of different serotypes of avian leukemia viruses
ActiveCN103074447AGood amplification effectReduce time to differential typingMicrobiological testing/measurementDNA/RNA fragmentationLeucosisSerotype
The invention discloses establishment of a PCR-HRM analysis method for rapid differential diagnosis of different serotypes of avian leukemia viruses. According to the invention, universal primers are good in degeneracy, have very good amplification performance to the A, B, C, D and E subtypes of avian leukemia viruses, is conductive to improving the efficiency of PCR (polymerase chain reaction), and reduces the time of identification and typing of viruses; and specific primers provided by the invention are good in specificity, and the J subtype can be amplified in a specific way. HRM analysis is performed on the amplification product obtained by using the primers, so that the typing of avian leukemia viruses can be performed accurately and rapidly in a high-throughput way by being compared with the standard HRM curve of a known subtype, particularly the endogenous type of avian leukemia viruses and the exogenous type of avian leukemia viruses can be rapidly and accurately distinguished.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
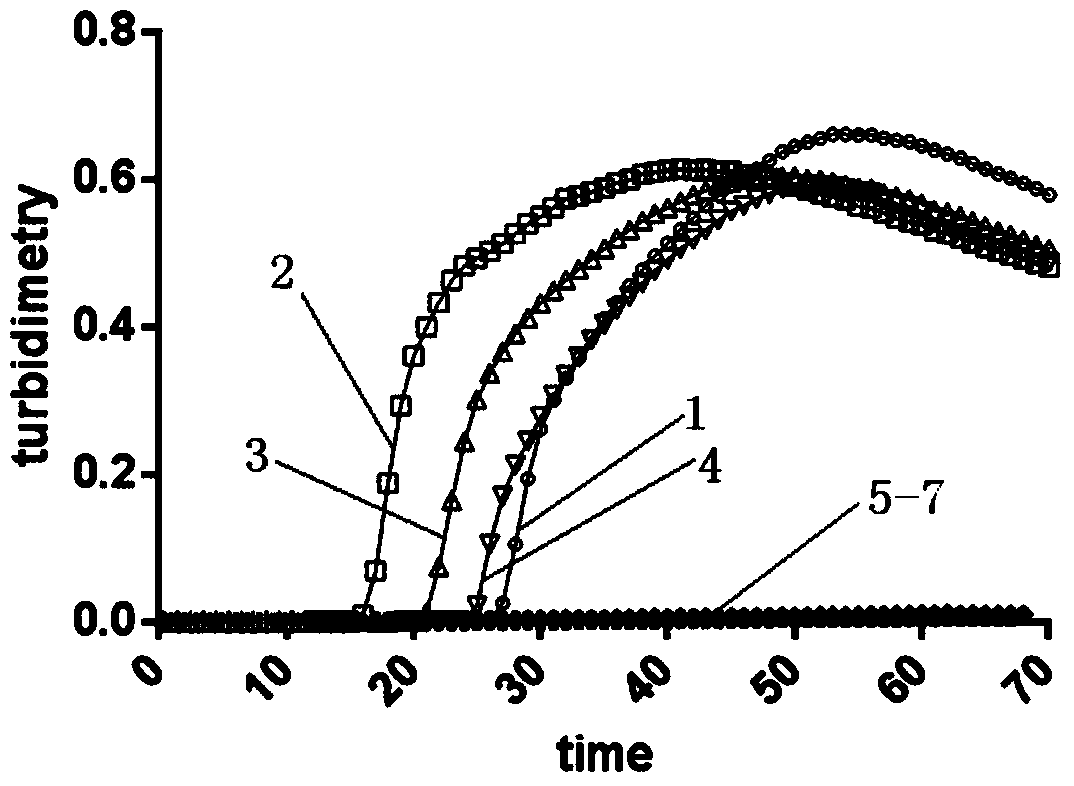
LAMP (Loop-Mediated Isothermal Amplification) kit for detecting main subtype avian leukemia virus
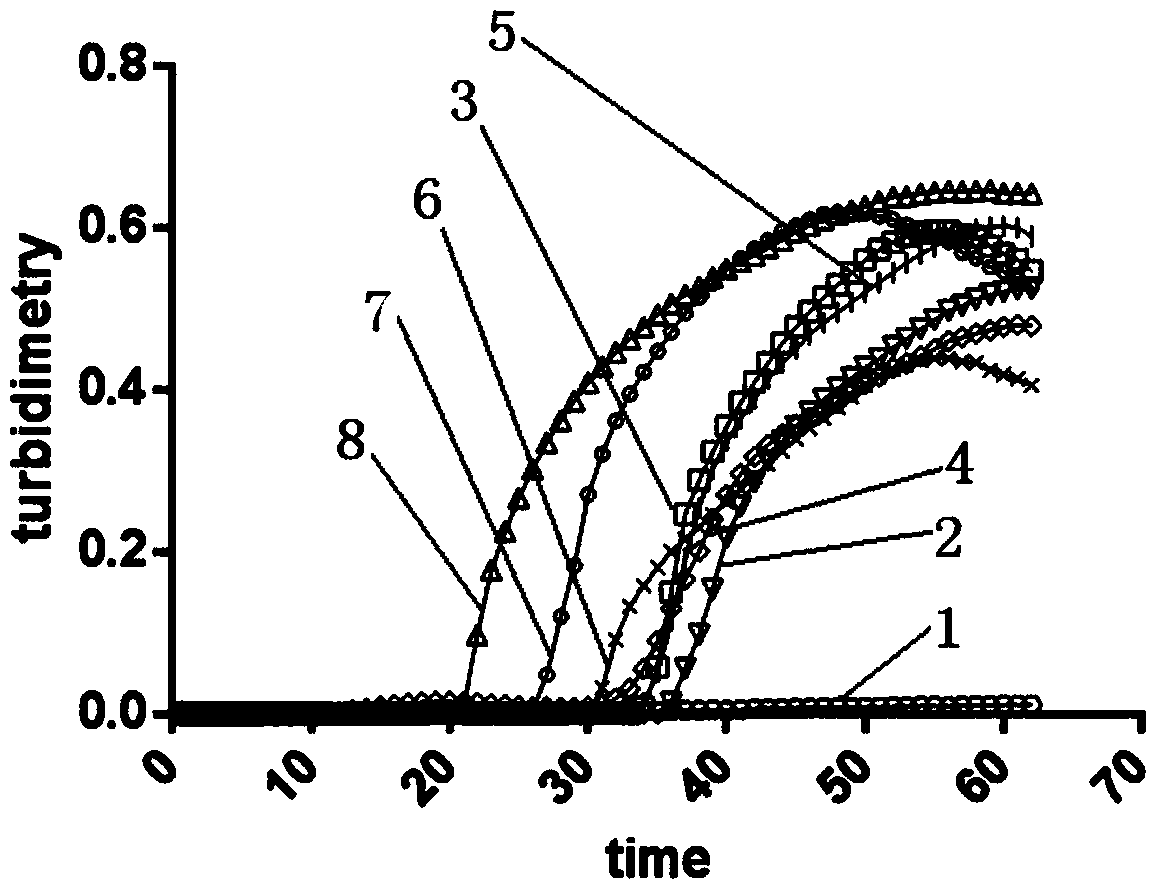
The invention discloses an LAMP (Loop-Mediated Isothermal Amplification) kit for detecting a main subtype avian leukemia virus. A loop-mediated isothermal amplification (LAMP) technology is adopted, and two pairs of specific primers (inner primers FIP and BIP, outer primers F3 and B3) are designed according to a POL (Point Of Load) gene sequence of the avian leukemia virus. By applying the LAMP kit and a detecting method established by the LAMP kit, an LAMP real-time turbidity meter is utilized to carry out real-time, quantitative and whole-course sealed monitoring analysis on LAMP reaction primers of the ALV (Avian Leukemia Virus), a reaction system and a reaction process, so that LAMP primers of the ALV are efficiently and specifically amplified. The LAMP kit disclosed by the invention has the advantages that the specificity is strong, the sensitivity is high, a result is quickly obtained, pollution is not caused and a product can be detected in real time; the avian leukemia virus can be detected out in real time by sampling according to the established system, and the detected result can be quickly and accurately obtained, so that convenience is brought for simply and quickly detecting the avian leukemia virus.
Owner:GUANGXI UNIV
Dual fluorescence quantification RT-PCR detection kit and application thereof
ActiveCN102230023AIncreased biosecurity riskPerfecting flu testingMicrobiological testing/measurementFluorescence/phosphorescenceSequence analysisReverse transcriptase
The invention provides a dual fluorescence quantification RT-PCR (reverse transcription-polymerase chain reaction) detection kit, comprising a deoxynucleoside triphosphate mixture, MgCl2, an RNA enzyme inhibitor, a Moloney murine leukemia virus reverse transcriptase, a DNA polymerase, a influenza virus standard and a reference substance. Based on sequence analysis of present pervasive A H1N1 influenza virus, the invention provides a multiple fluorescence quantification PCR molecular biology gene diagnosis method and a diagnostic kit which are rapid, specific, accurate and sensitive. In addition, one reaction tube can simultaneously detect and differentiate an influenza A virus or an influenza B virus in 2h, so as to improve influenza detection.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Method for labeling antibodies by colorful fluorescent granules and test paper strip prepared from antibodies
InactiveCN107132348AThe result is accurateRealize qualitative and quantitative simultaneous detectionBiological material analysisAnimal virusLeucosis
The invention provides a method for labeling antibodies by colorful fluorescent granules and a test paper strip prepared from the antibodies. Animal viruses including, but not limited to, canine distemper viruses, canine parvoviruses, canine adenoviruses, canine coronavirus, rabies viruses, feline leukemia viruses, Marek's disease viruses, Newcastle disease viruses and the like are used as targets, and the corresponding antibodies labeled by colorful fluorescent micro-spheres are prepared by the aid of chemical covalent processes and can be applied to detecting the animal viruses. The method for labeling the antibodies by the colorful fluorescent granules and the test paper strip prepared from the antibodies have the advantages that colorful detection lines with bright developed colors can appear during detection, and accordingly qualitative results can be obtained; the contents of the animal viruses in samples further can be subsequently quantitatively obtained, accordingly, test results are clear and are high in stability, and the test paper strip can be stored at the room temperature for a long time.
Owner:江苏雷森生物科技有限公司
Immune PCR reagent kit for detecting avian leukemia virus
InactiveCN105779649AMicrobiological testing/measurementMicroorganism based processesAntigenOrtho position
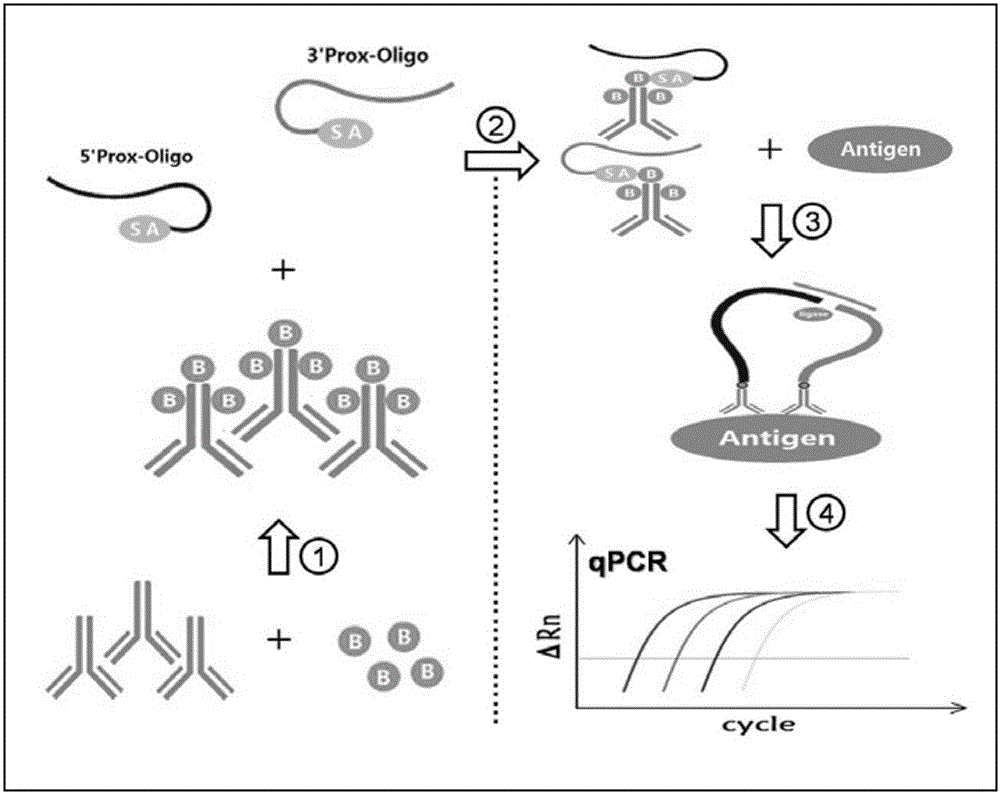
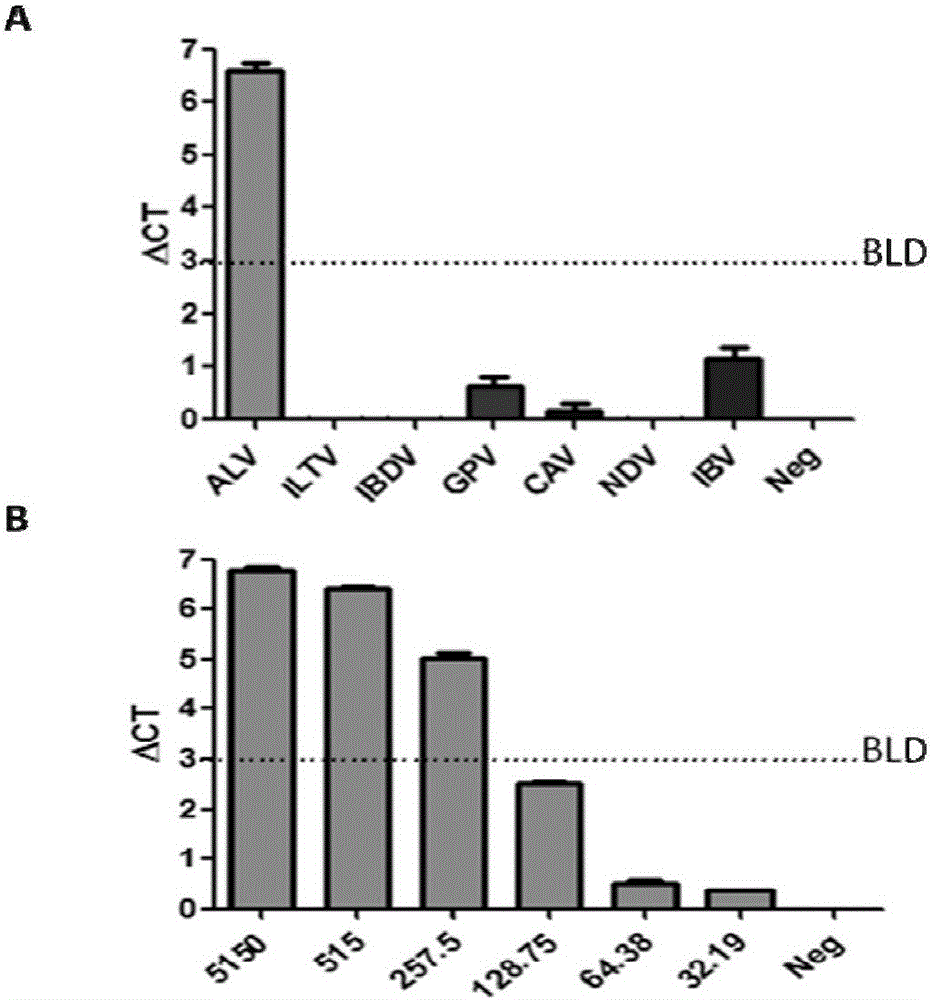
The invention belongs to the field of biological technical detection and particularly relates to an immune PCR reagent kit for detecting avian leukemia virus. The reagent kit comprises two antibody oligonucleotide probes, a coupled reaction solution, DNA ligase, protease, a Fast Master mixed solution and a Universal q PRC reaction solution. The immune PCR reagent kit uses a biotin-labeled avian leukemia virus preventing special antibody and a commercialized ortho-position connecting reagent, can detect avian leukemia virus antigen through immune PCR and can quickly detect the avian leukemia virus antigen in a high-throughout mode through the immune PCR without carrying out a complex nucleic acid extracting process on a sample. The immune PCR reagent kit for detecting the avian leukemia virus can be applied to clinical detection and purification of avian leukemia virus and will fill domestic and oversea correlated technique blank.
Owner:YANGZHOU UNIV +1
Method of treatment for feline leukemia virus infections
InactiveUS6350443B1Reduce feverReduce in quantityBiocidePeptide/protein ingredientsGranulocytopeniasInterferon alpha
A method of treatment for feline leukemia virus infections by continuously administering a feline interferon preparation containing a feline interferon as a main component daily to a cat is disclosed. As a feline interferon, a feline omega (omega)-interferon is preferably used, and more particularly, a recombinant interferon is preferably used. A method of treatment using a therapeutic agent containing a feline omega-interferon as a main component in accordance with the present invention is a novel and superior method suitable for treating feline leukemia virus infections, and in particular, for treating neutropenia.
Owner:TORAY IND INC
Preparation method and application of avian leukemia virus antigen immunosensor
InactiveCN104459123AIncrease the amount of detectionEasy to separateMaterial analysis by electric/magnetic meansImmune profilingCyclodextrin
The invention belongs to the technical field of immunoassay and bio-sensing, and discloses a preparation method and an application of an avian leukemia virus antigen immunosensor which is used for quickly detecting the avian leukemia virus antigen. According to the manufacturing scheme adopted by the invention, the preparation method comprises the following steps: by taking a glassy carbon electrode as a working electrode, modifying beta-CD / MWCNTs; then, adding adamantanecarboxylic acid / capturing antibody Ab1, bovine serum albumin, avian leukemia virus antigen as well as beta-CD@Fe3O4@SiO2-Fc / Ab2 solution in sequence. The beta-CD@Fe3O4@SiO2-Fc is easy to separate due to the superparamagnetism of a ferric oxide nano material; by utilizing the supramolecular recognition characteristic of the cyclodextrin, a great deal of ferrocenecarboxylic acid Fc can be captured through the action of subjective and objective objects, the biocompatibility and the water solubility are good, so that the preparation method is beneficial for increasing the antibody detecting amount, capable of realizing relatively high sensitivity and lowering the detecting limit to 0.33pg / mL.
Owner:UNIV OF JINAN
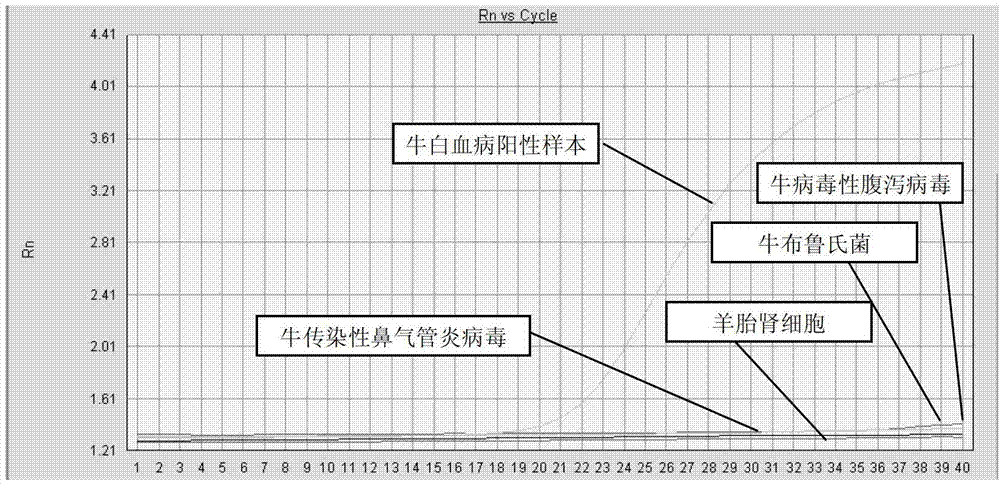
Kit for detecting proviral DNA (deoxyribonucleic acid) of bovine leukemia virus (BLV) and application of kit
InactiveCN103045762AEasy to makeThe method is simple and fastMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention provides a kit for detecting proviral DNA (deoxyribonucleic acid) of bovine leukemia virus (BLV). The kit comprises (a) fluorescent PCR (polymerase chain reaction) liquid reactant, (b) a fluorescent probe, (c) hot start Tag enzyme, (d) a standard positive template and (e) negative control, wherein the fluorescent PCR liquid reactant contains forward primers and reverse primers; the sequence of the forward primers is shown in SEQ ID NO:2; the sequence of the reverse primers is shown in SEQ ID NO:3; the sequence of the fluorescent probe is shown in SEQ ID NO:4; fluorescent substance is designed at the 5' end of the sequence of the fluorescent probe; cancellation substance is designed at the 3' end of the sequence of the fluorescent probe; and the nucleotide insertion sequence of the standard masculine template is shown in SEQ ID NO:5. The invention further provides application of the kit to detecting proviral DNA of BLV in whole blood. The PCR detection kit provided by the invention can be used for quickly and accurately detecting BLV and is applicable to quick diagnosis of BLV, epidemiological investigation and risk assessment.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
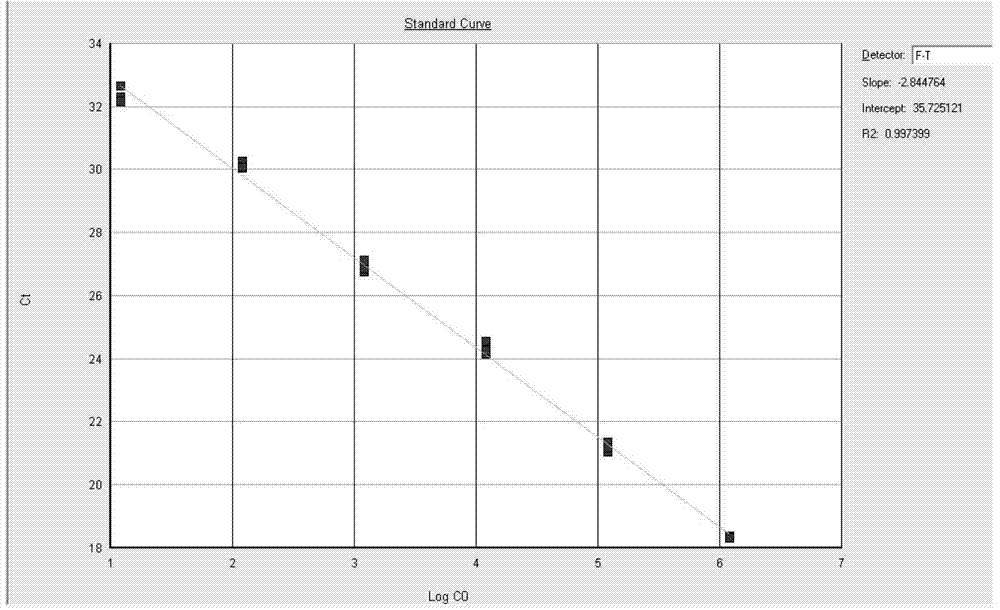
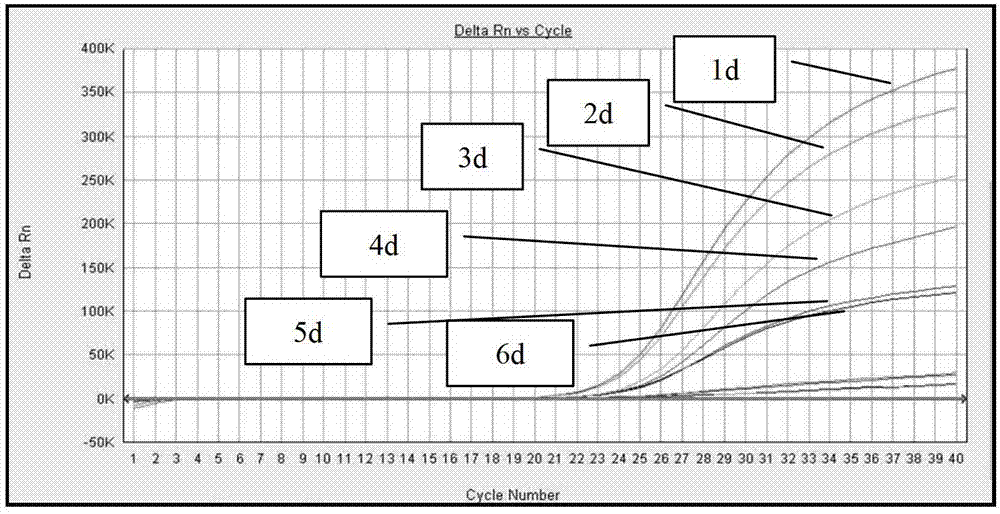
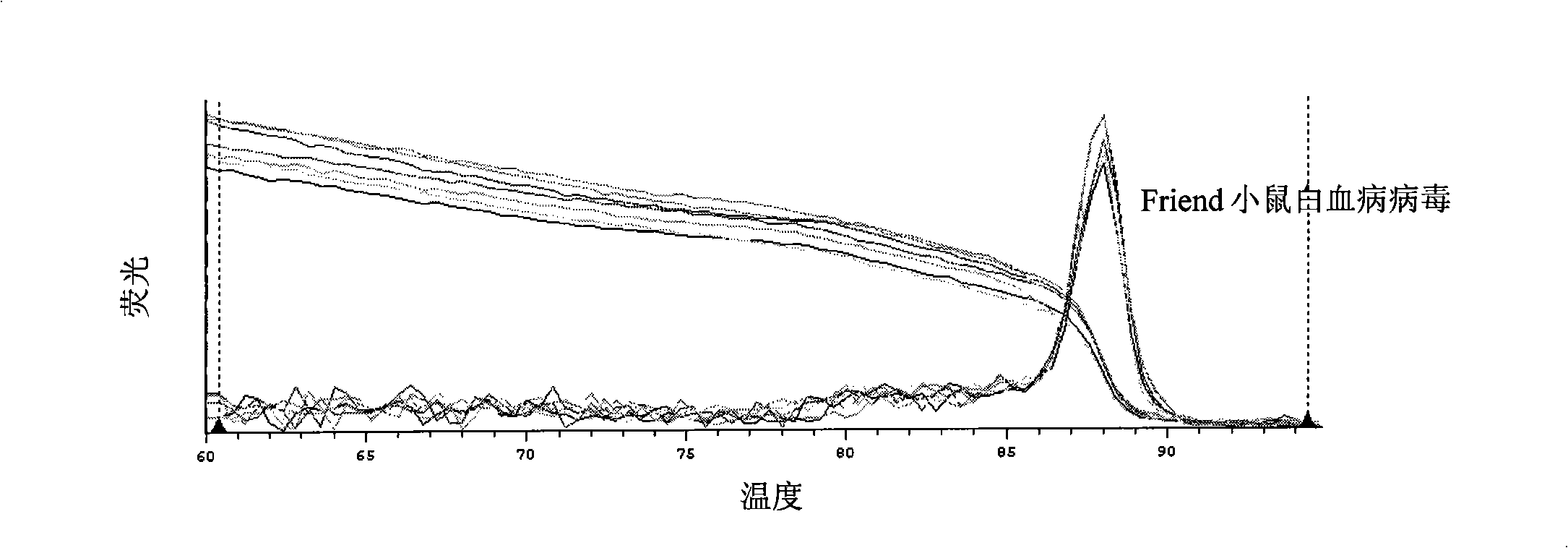
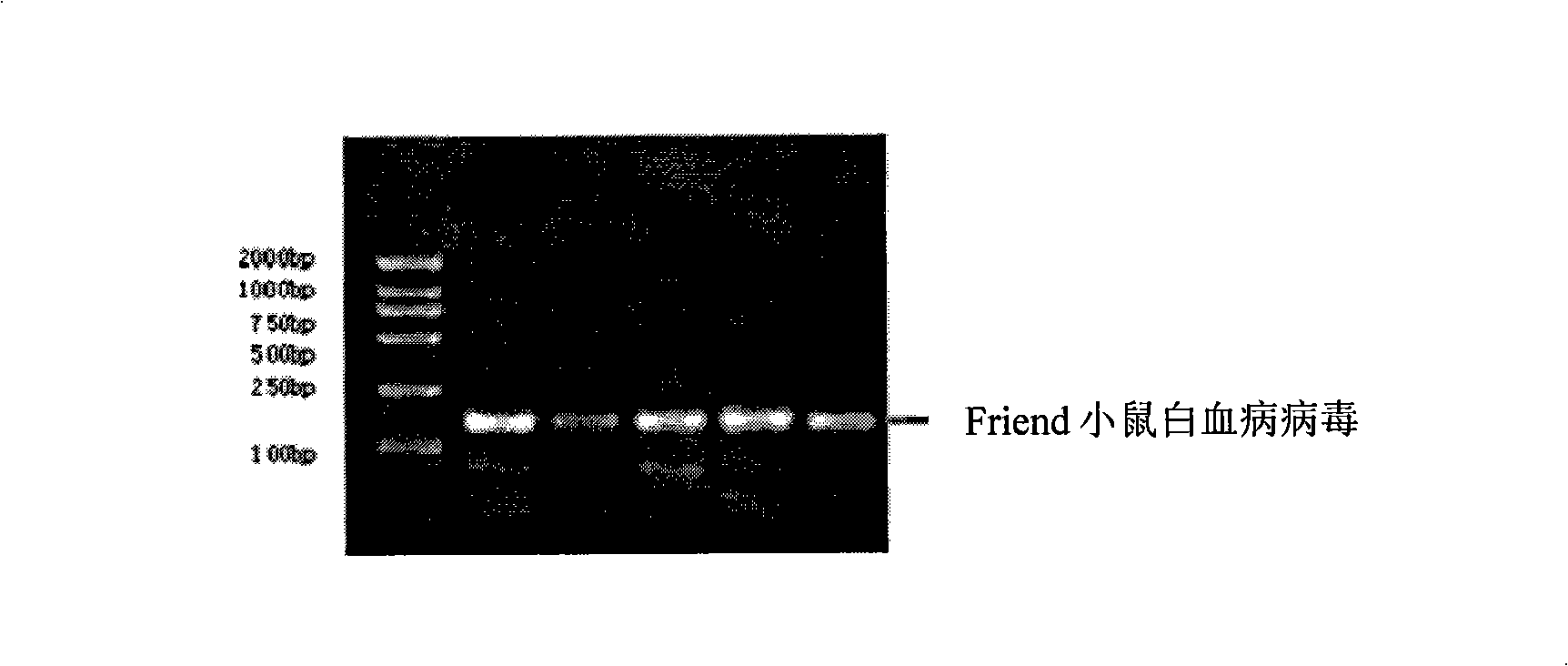
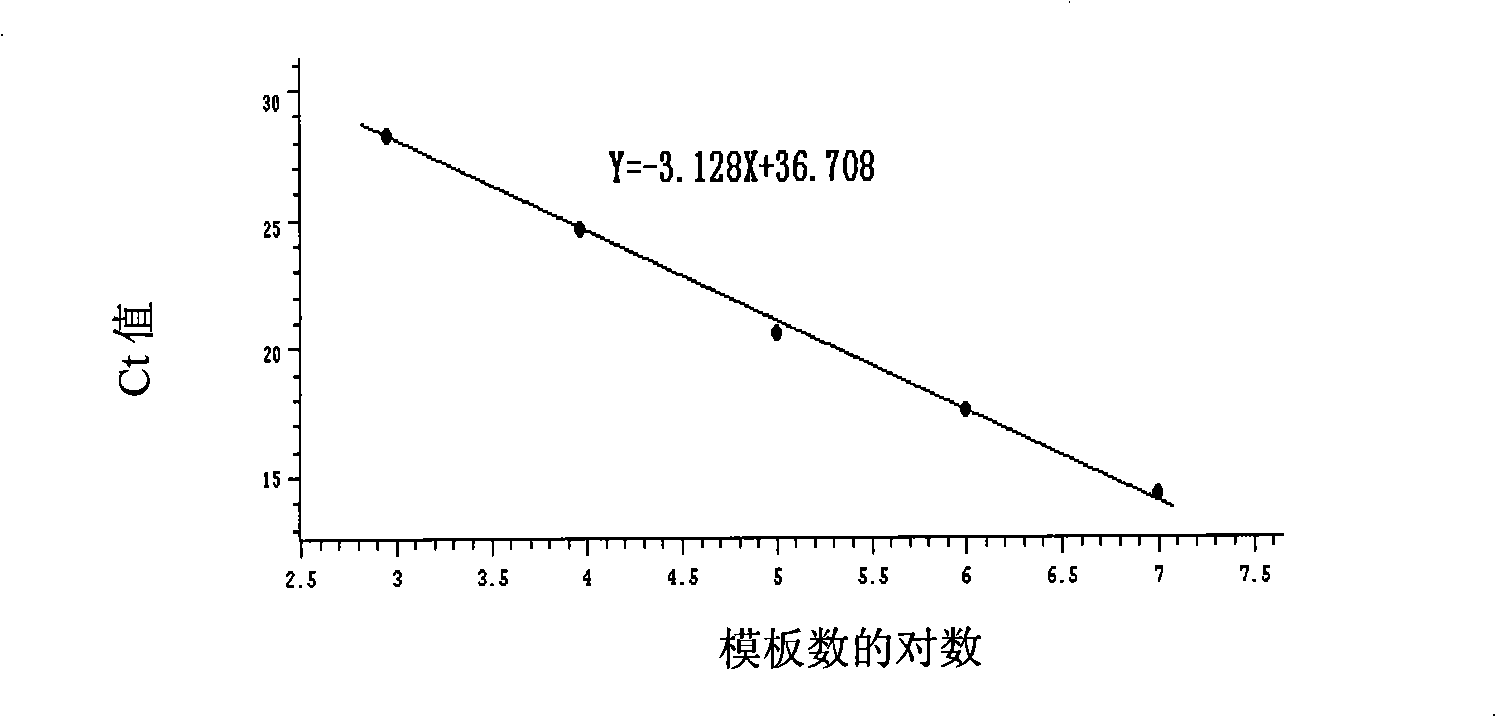
Standard article and method for detecting carry quantity of leucovirus
The invention discloses a standard product which is used for detecting the viral load of Friend murine leukemia, and a method which detects the viral load of the Friend murine leukemia by a real-time fluorescent quantitative polymerase chain reaction method (Real-Time RT-PCR method) by using the standard product. The detection process includes the steps of the extraction and content measurement of leukovirus nucleic acid (RNA), the obtaining of the nucleic acid segment by amplifying reverse transcription by using a primer, the detection of the real-time fluorescent quantitative polymerase chain reaction (Real-time PCR), and the like. Compared with conventional PCR detection methods, the method of the invention, which detects the viral load of the Friend murine leukemia, has better specificity. The detected genetic amplified products are target genetic products to be detected. The method has a better linear relationship and is suitable for being applied to the detection of the viral load of the Friend murine leukemia (Fr. MuLV).
Owner:崔晓兰
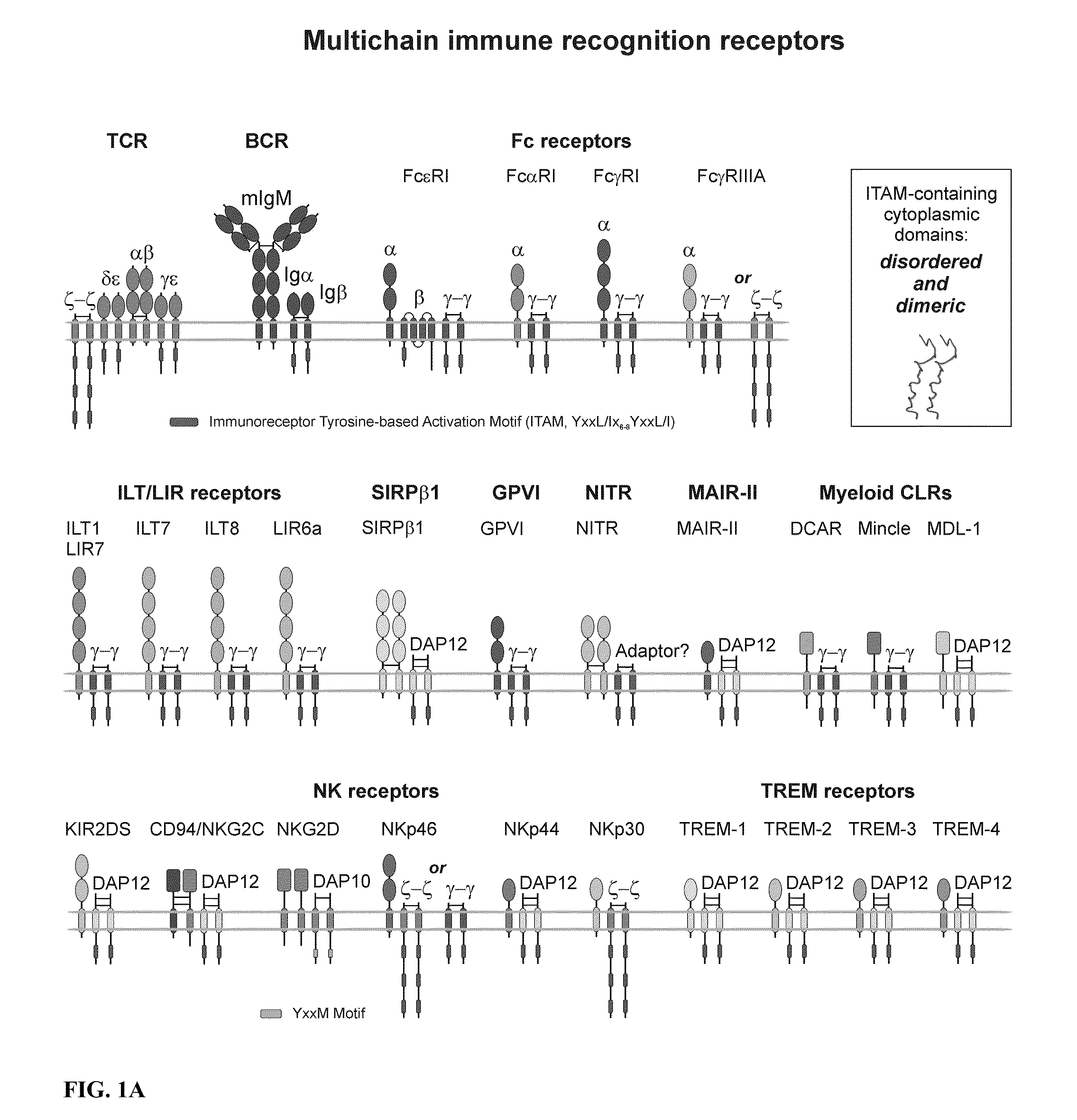
Inhibition of TCR Signaling with Peptide Variants
ActiveUS20130039948A1Current is limitedIn-vivo radioactive preparationsVirus peptidesADAMTS ProteinsT cell
The present invention provides compositions comprising peptides derived from amino acid sequences (or from combinations thereof) of fusion and other protein regions of various viruses, including but not limited to, severe acute respiratory syndrome coronavirus, herpesvirus saimiri, human herpesvirus 6, Lassa virus, lymphocytic choriomeningitis virus, Mopeia virus, Tacaribe virus, Friend murine leukemia virus; human T lymphotropic virus type 1; herpesvirus ateles; Marburg virus; Sudan Ebola virus; Zaire Ebola virus, and comprising L- and / or D-amino acids and combinations thereof, which affect T cells by acting on the T cell antigen receptor (TCR). More specifically, the peptides act on the TCRαβ-CD3δε-CD3γε-ζζ signaling complex. Yet more specifically, the peptides act on the TCRα / CD3δε / ζζ signaling module of TCR. The present invention further relates to the prevention and therapy of various T cell-related disease states involving the use of these compositions. Specifically, the compositions are useful in the treatment and / or prevention of a disease or condition where T cells are involved or recruited. The compositions of the present invention also are useful in the production of medical devices comprising peptide matrices (for example, medical implants and implantable devices).
Owner:SIGNABLOK
Nucleobase phosphonate analogs for antiviral treatment
ActiveUS7579332B2Increasing cellular accumulation and retentionIncrease valueBiocideOrganic active ingredientsViral Reverse TranscriptionHuman T cell leukemia virus
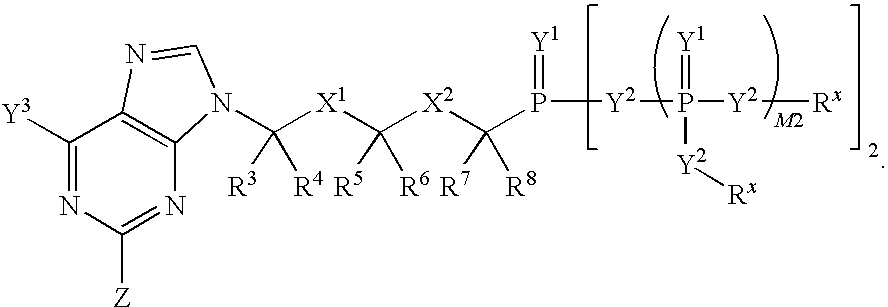
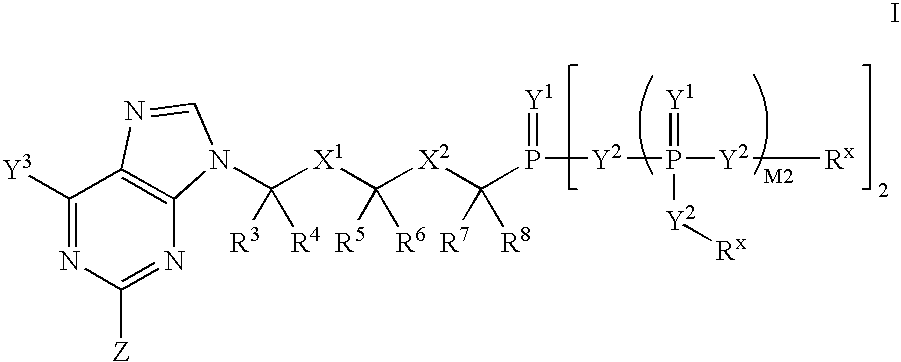
The invention provides compounds with activity against infectious diseases. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication, of the virus. The compounds of the invention may be useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-l or HIV-2) or human T-cell leukemia virus (HTLV-1 or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. Representative of the invention is a compound of the following formula, with the substituents defined herein:
Owner:GILEAD SCI INC
Short corynebacteria pharmaceutics or short corynebacteria pharmaceutics without cells in application for preparing medicine of curing HIV infection or AIDS
ActiveCN1600323AGuaranteed pollutionPromote absorptionBacteria material medical ingredientsAntiviralsTraditional medicineLeukemogenic Viruses
An application of the CP or NCP preparation in preparing the medicine for treating HIV infection or AIDS is disclosed. Said CP preparation is prepared from CP through deactivating by formaldehyde.
Owner:上海昌润生物科技有限公司
Application of seaweed oligosaccharide in preparation of avian leucosis virus resistant preparation
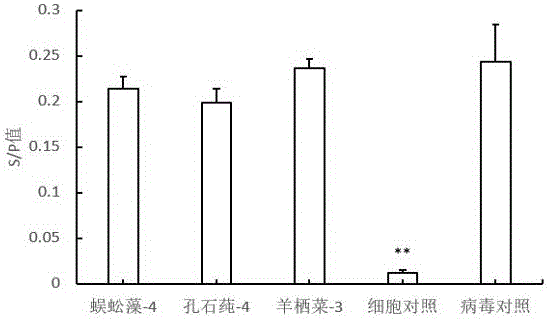
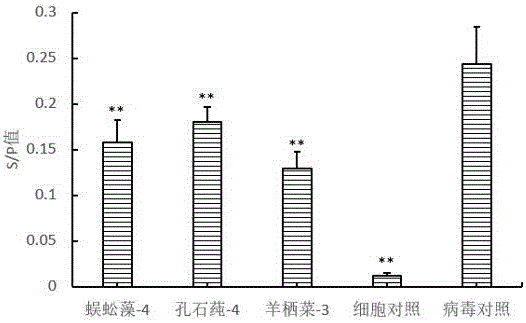
ActiveCN106727623AAbundant resourcesImprove immunityOrganic active ingredientsAntiviralsLeucosisSARGASSUM FUSIFORME
The invention belongs to the technical field of biomedicine and particularly relates to application of seaweed oligosaccharide in the preparation of an avian leucosis virus resistant preparation. seaweed oligosaccharide is prepared by degrading natural polysaccharides extracted from the macroalga Grateloupia filicina, Ulva lactuca, Sargassum fusiforme and other algae, has a wide source range, is simple to prepare, and has the advantages, as an antiviral preparation, such as naturalness, zero toxicity and environmental friendliness; in-vitro cell experiments prove that the seaweed oligosaccharide is capable of inhibiting the proliferation of avian leucosis virus, has significant antiviral effect, may be applied to livestock and poultry breeding as a novel antiviral preparation, and has high applicable value.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
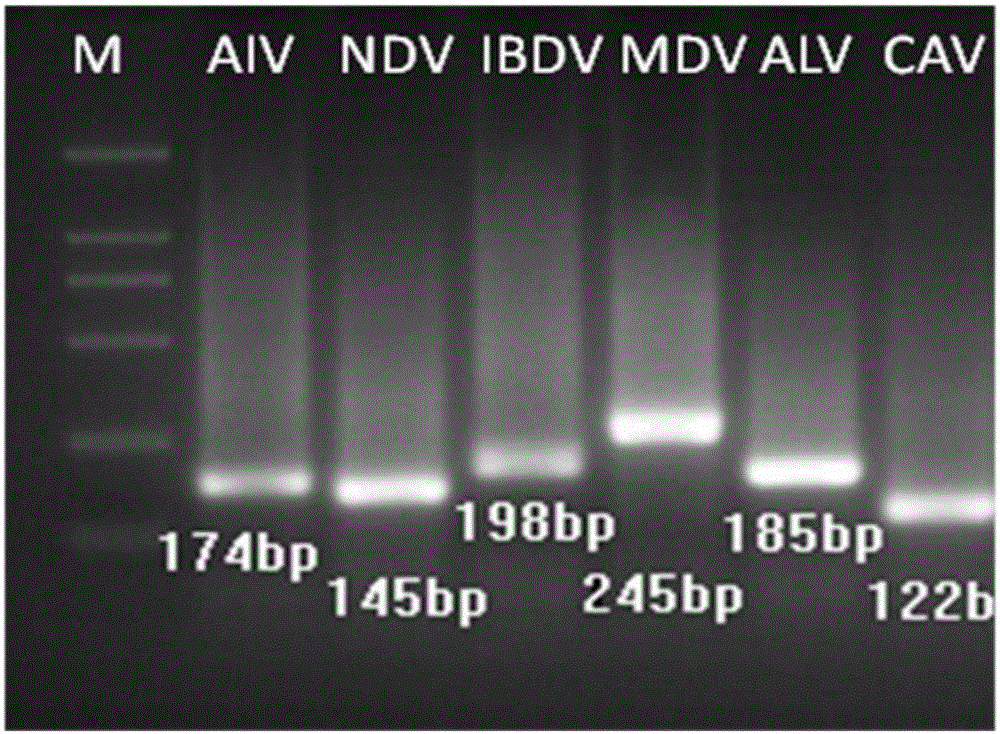
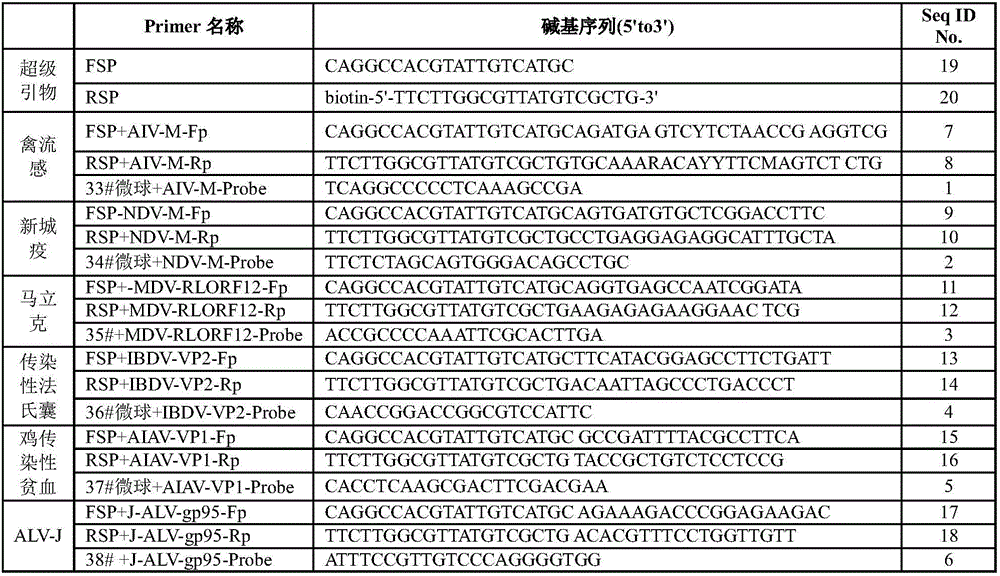
Multiplex detection reagent for immunosuppressive poultry pathogens of AVI (avian influenza) and the like and application of multiplex detection reagent
ActiveCN106480173AQuick checkFast detection methodMicrobiological testing/measurementNewcastle disease virus NDVMarek's disease
The invention discloses a multiplex detection reagent for immunosuppressive poultry pathogens of avian influenza and the like and an application of the multiplex detection reagent. The multiplex detection reagent comprises six specific probes with sequences shown in Seq ID NO.1-Seq ID NO.6 which refer to AVI virus, ND (newcastle disease) virus, serotype I Marek's disease virus, IBDV (infectious bursal disease virus), avian infectious anaemia virus and ALV-J (aivan leukosis virus subgroup J) in sequence. The reagent comprises the specific probes for six immunosuppressive poultry pathogens comprising AVI and the like and can specifically capture target sequences of the six immunosuppressive poultry pathogens, and the foundation is laid for high-throughput rapid detection of the six immunosuppressive poultry pathogens. With adoption of the reagent, a rapid and accurate detection method is provided for the six immunosuppressive poultry pathogens on the basis of a liquid chip.
Owner:SHENZHEN CUSTOMS ANIMAL & PLANT INSPECTION & QUARANTINE TECH CENT +2
Detection method for rapidly and definitely diagnosing feline leukemia and preparation of test strip thereof
InactiveCN106841608AThe result is accurateShorten detection timeBiological material analysisCouplingRoom temperature
The invention provides a detection method for rapidly and definitely diagnosing feline leukemia and a preparation method of a test strip thereof. Feline leukemia virus (abbreviated as FeLv) is used as a target to prepare the corresponding test strip by virtue of a chemical covalent coupling method, and the test strip is applied to the detection of the feline leukemia virus by utilizing a fluorescent labeled immnochromatography assay. The test strip for rapidly detecting the feline leukemia virus provided by the invention is high in sensitivity; a negative / positive result can be obtained, the content of the feline leukemia virus in a sample can also be quantitatively obtained, the test result is clearer and relatively high in stability, and the test strip can be stored for a long time at a room temperature.
Owner:江苏雷森生物科技有限公司
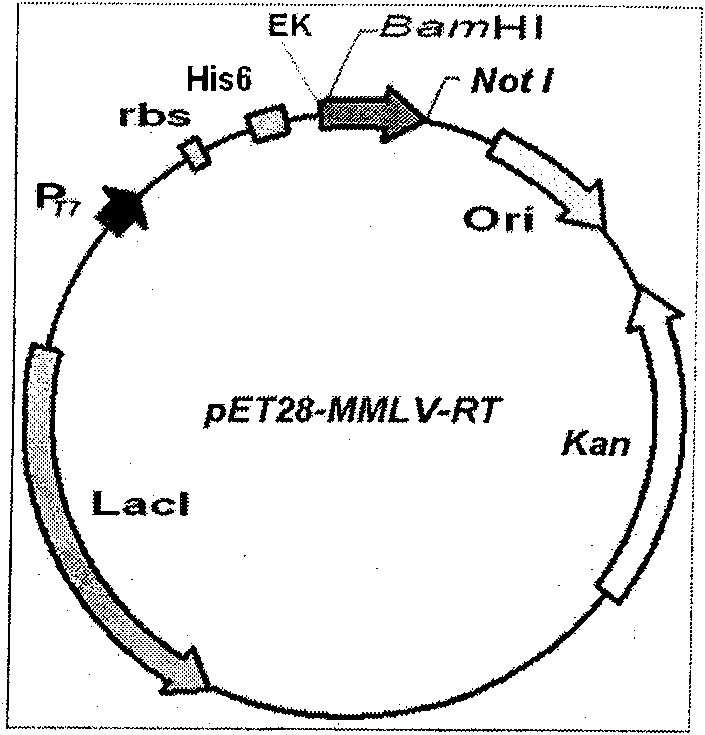
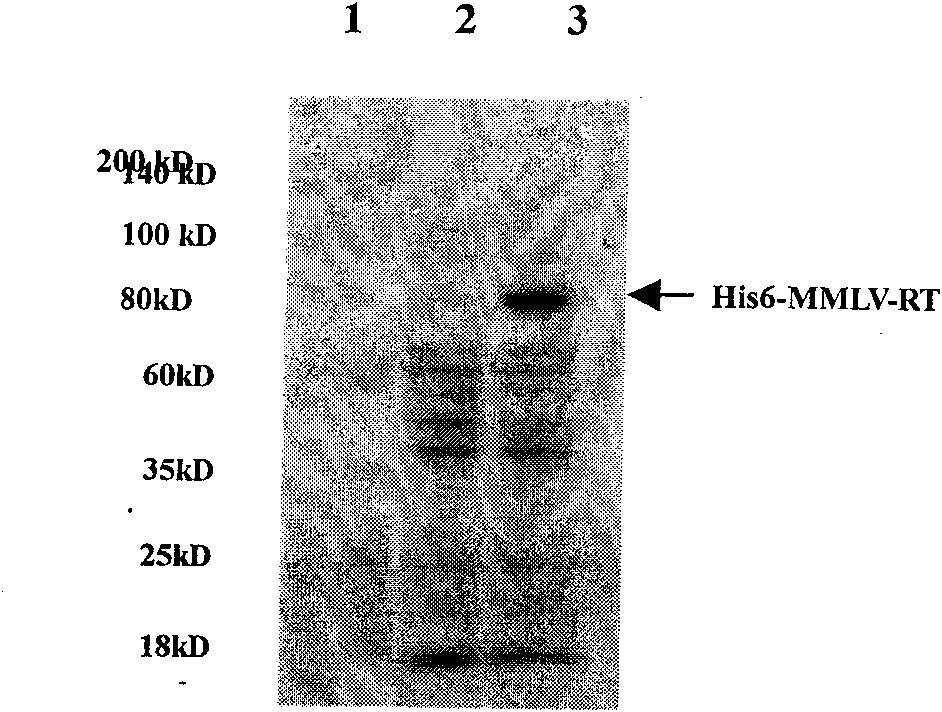
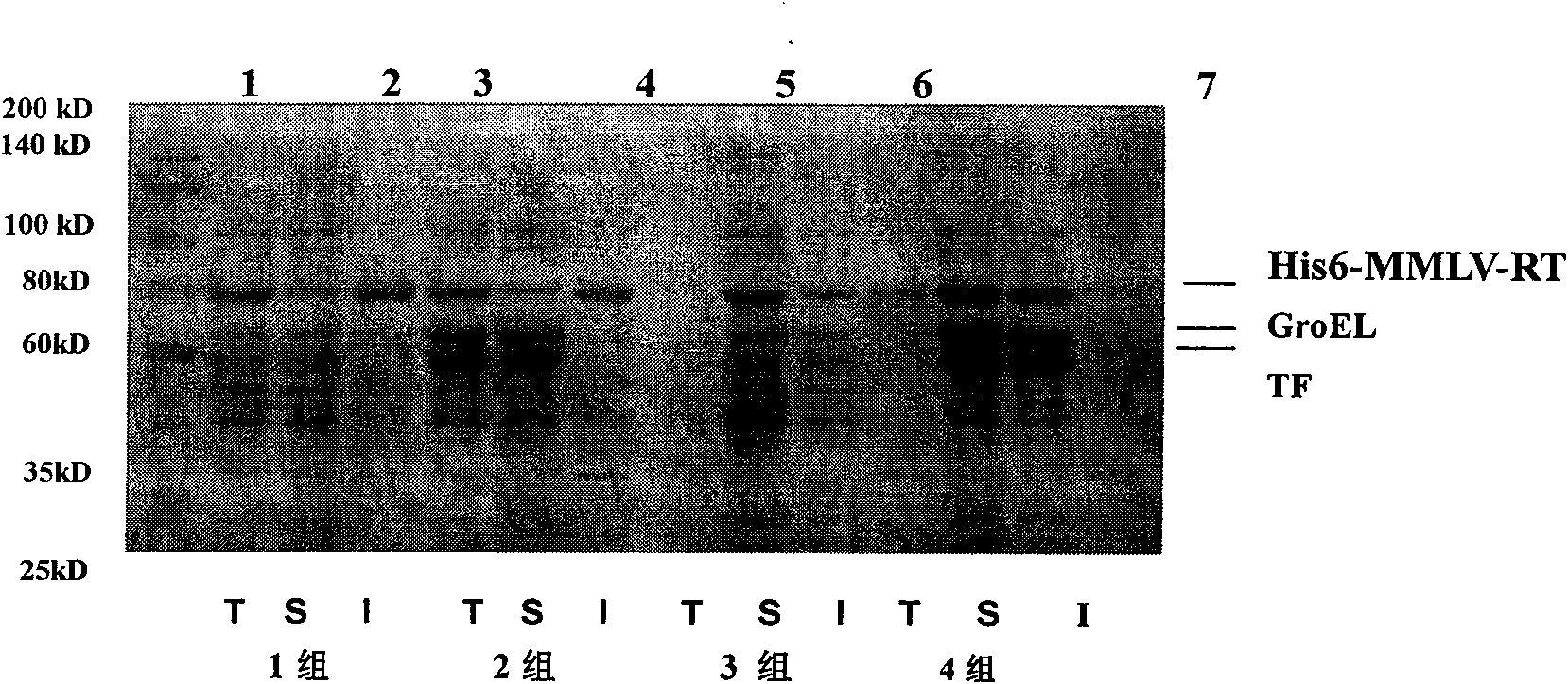
Soluble expression and purification method for recombinant murine leukemia virus reverse transcriptase (MMLV-RT) in colon bacillus
InactiveCN101684474AImprove solubilityEasy to purifyMicroorganism based processesEnzymesRestriction Enzyme Cut SiteSolubility
The invention relates to an expression and purification of murine leukemia virus reverse transcriptase (MMLV-RT), in particular to a soluble expression and a purification method for recombinant murineleukemia virus reverse transcriptase (MMLV-RT), which belongs to the field of biochemistry. MMLV-RT genes and enterokinase restriction enzyme cutting sites are cloned in a pET28a vector through vector construction; the MMLV-RT and molecular chaperones are co-expressed at the low temperature; the expression amount of protein is improved to 15 percent; and the solubility of the protein is 10 timesthat of the protein expressed by a conventional method.
Owner:孙启明 +1
Method for detecting nucleic acid of porcine reproductive and respiratory syndrome virus in one step
InactiveCN102181580AThe solution is not easy to saveSolve transportation problemsMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseCentral laboratory
The invention relates to a method for detecting nucleic acid of a porcine reproductive and respiratory syndrome (PRRS) virus by one step, which comprises the following steps of: collecting, processing and detecting samples, wherein in the collecting step, an animal blood sample is dripped into a full type approval (FTA) card sample area; and the detecting steps comprises the: (1) designing specific primers and a probe for general type PRRS virus, and marking carboxyfluorescein (FAM) and tetramethyl rhodamine (TAMARA) fluorescent groups on the probe; and (2) preparing and optimizing a detection system and a reaction condition, wherein a reaction system comprises 25 or 50 microliters of trihydroxymethyl aminomethane-hydrogen chloride (HCl) (the pH value is between 7.8 and 9.0), 0.1 to 0.5 micro mol of upstream primer and 0.1 to 0.5 micro mol of downstream primer, 100 to 400 micro mols of deoxynucleotide mixture, 0.1 to 0.5 micro mol of probe, 100 to 300 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase, 1 to 5 U of thermostable deoxyribonucleic acid (DNA) polymerase, 4 to 8 mols of Mg<2+->, 300 to 500 nano mol of homogenized reference dyes ROX, and 1 to 15 microliters of sample which is re-suspended in trihydroxymethyl aminomethane-ethylene diamine tetraacetic acid (EDTA) buffer solution and is added before each time of reaction. In the method, the sample collection is easy and convenient, so that an FTA card containing s ribonucleic acid (RNA) sample can be posted to any one central laboratory to be detected according to a form of regular mails; and the pollution risks are reduced, and the detection sensitivity is improved.
Owner:湖南农安生物技术有限公司
Multi-PCR (Polymerase Chain Reaction) primer group, kit and method for detecting A, B, J and K subgroups avian leukosis viruses
ActiveCN109055615AStrong specificityReduce mutual interferenceMicrobiological testing/measurementMicroorganism based processesPositive controlAvian leukosis viruses
The invention discloses a multi-PCR (Polymerase Chain Reaction) primer group for detecting A, B, J and K subgroups avian leukosis viruses (ALV). The multi-PCR primer group comprises a common upstreamprimer SEQ ID NO. 1 for detecting the avian leukosis virus, a downstream primer SEQ ID NO. 2 of the A subgroup avian leukosis virus, a downstream primer SEQ ID NO. 3 of the B subgroup avian leukosis virus, a downstream primer SEQ ID NO. 4 of the K subgroup avian leukosis virus and a downstream primer SEQ ID NO. 5 of the K subgroup avian leukosis virus. The invention further discloses a multi-PCR kit for detecting the A, B, J and K subgroups avian leukosis viruses; the multi-PCR kit comprises the multi-PCR primer group provided by the invention, Premix Ex Taq DNA (Deoxyribonucleic Acid) polymerase, sterile double distilled water and a negative and positive control plasmid DNA template. Furthermore, the invention further discloses a multi-PCR method for detecting the A, B, J and K subgroupsavian leukosis viruses. An experiment shows that the multi-PCR primer group provided by the invention can be used for detecting the A, B, J and K subgroups avian leukosis viruses from a sample at thesame time, has the advantages of strong specificity, high sensitivity and convenience for operation and result judgment and is suitable for detecting batch samples and primary identification of exogenous ALV.
Owner:JIANGSU INST OF POULTRY SCI
Abl1 inhibitor for treating and preventing ocular neovascularisation
InactiveUS20170027936A1Inhibit angiogenesisInhibit cell migrationOrganic active ingredientsSenses disorderViral OncogeneVEGF receptors
The present invention relates to an Abelson murine leukaemia viral oncogene homolog 1 (ABL1) inhibitor for use in the treatment of ocular neovascularisation associated with a non-cancerous condition. The invention also relates to the use of an ABL1 inhibitor as a complementary therapy with VEGF or VEGF receptor inhibitor treatment and provides pharmaceutical compos itions and kits comprising one or both inhibitors.
Owner:UCL BUSINESS PLC
J substock lymphoid leuoosis-resistant polyclonal antibody and preparation method thereof
InactiveCN101863977AAvoid infectionWill not lose validitySerum immunoglobulinsImmunoglobulins against virusesConserved sequenceSmall peptide
The invention relates to a J substock lymphoid leuoosis-resistant polyclonal antibody and a preparation method thereof. The J substock lymphoid leuoosis-resistant polyclonal antibody can effectively inhibit the infection of J substock avian leukosis virus, and is obtained on the basis of highly conserved sequence synthesized small peptides formed by 27 amino acid in transmembrane protein coded with J substock avian leukosis virus gp 37 gene through a coupled keyhole hemocyanin immune rabbit; and the polyclonal antibody blocks the invasive J substock avian leukosis virus reproduction by being combined with the nucleus non-specifity, and is combined with and eliminates the specificity of the J substock avian leukosis virus, thereby effectively preventing the generation and spreading of the J substock lymphoid leuoosis, greatly reducing the economic loss of aviculture caused by infection of J substock avian leukosis virus, and providing a novel method and thinking for the lymphoid leuoosis preventing and controlling.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Method for blocking vertical transmission of avian leukosis virus and application of method
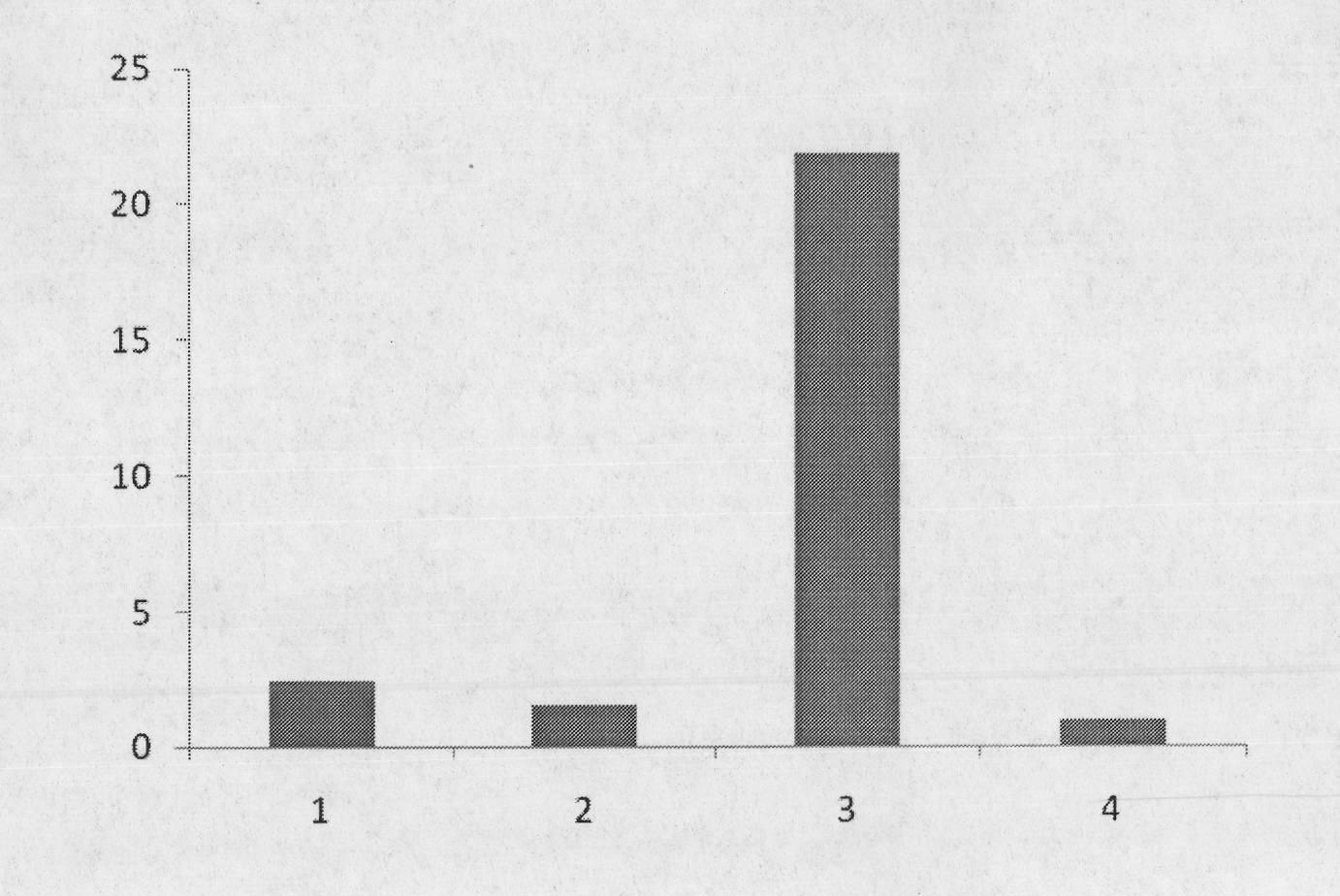
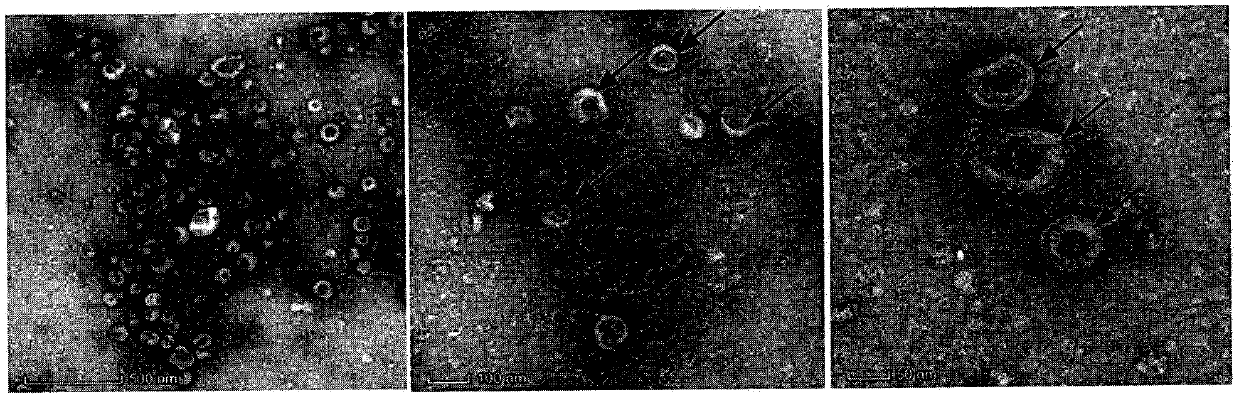
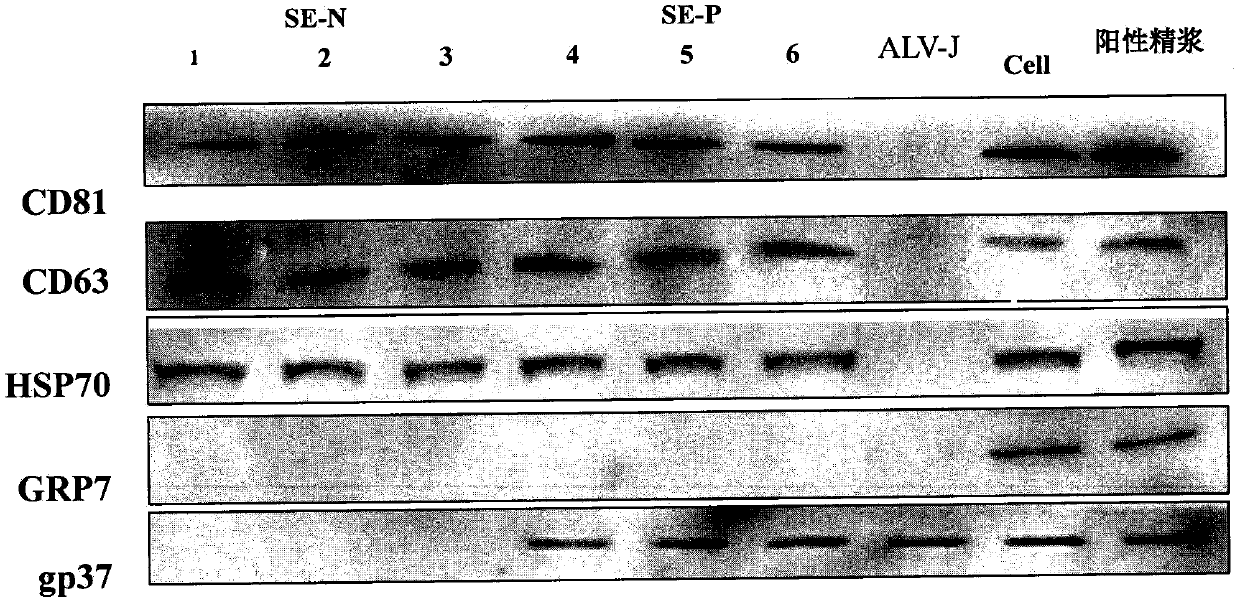
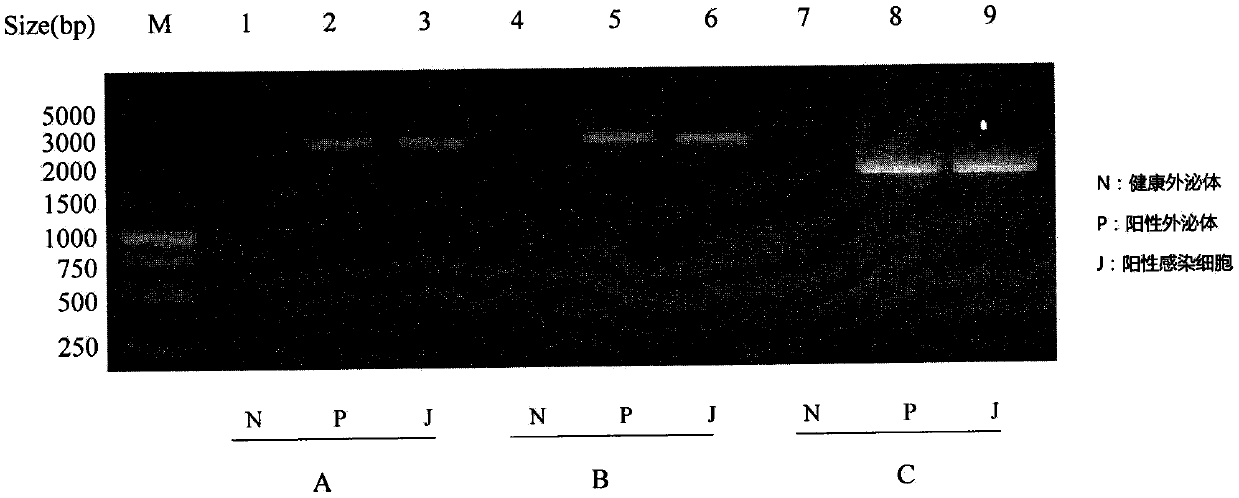
The invention discloses a method for blocking vertical transmission of avian leukosis virus and an application of the method. The inventor discovers for the first time that vertical transmission of avian leukosis virus is carried, mediated and infected by exosomes derived from a reproductive system of avian leukosis positive chickens to infect receptor chickens and progeny chicks, and provides a method and a blocking agent for blocking vertical transmission of ALV by interfering with seminal exosomes of the avian leukosis positive chickens. The method has the advantages that the exosomes related to the vertical transmission of the avian leukosis virus provide a new target point for treating or preventing the avian leukosis; and the provided blocking method is not affected by virus strain subpopulation and variation, is uniformly cut off from the transmission route, has a wide range of targets, is simple and rapid, and has a good effect.
Owner:SOUTH CHINA AGRI UNIV
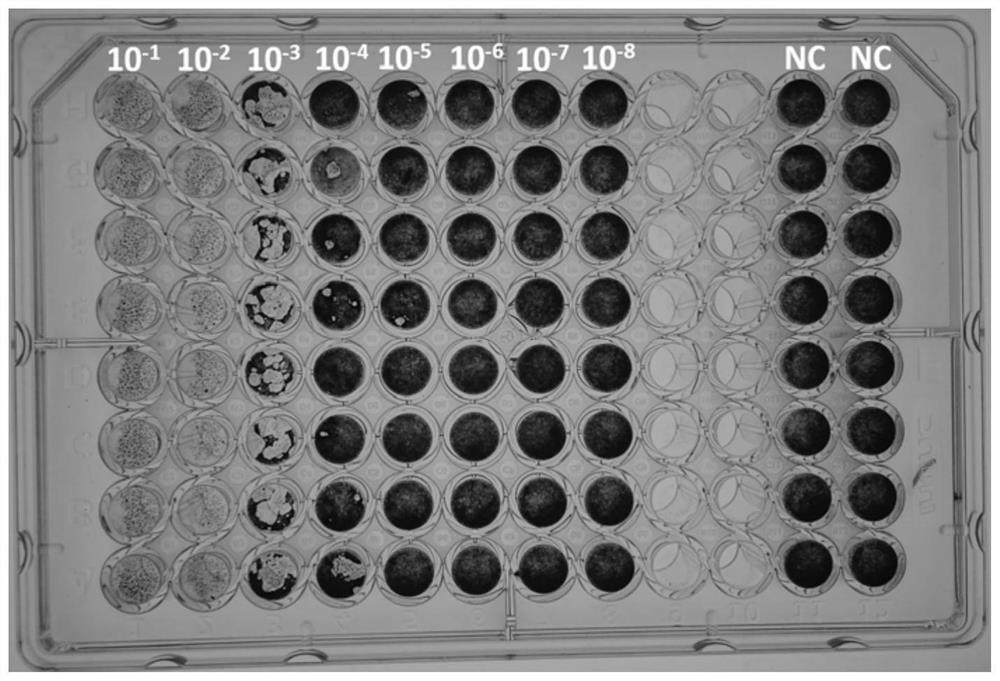
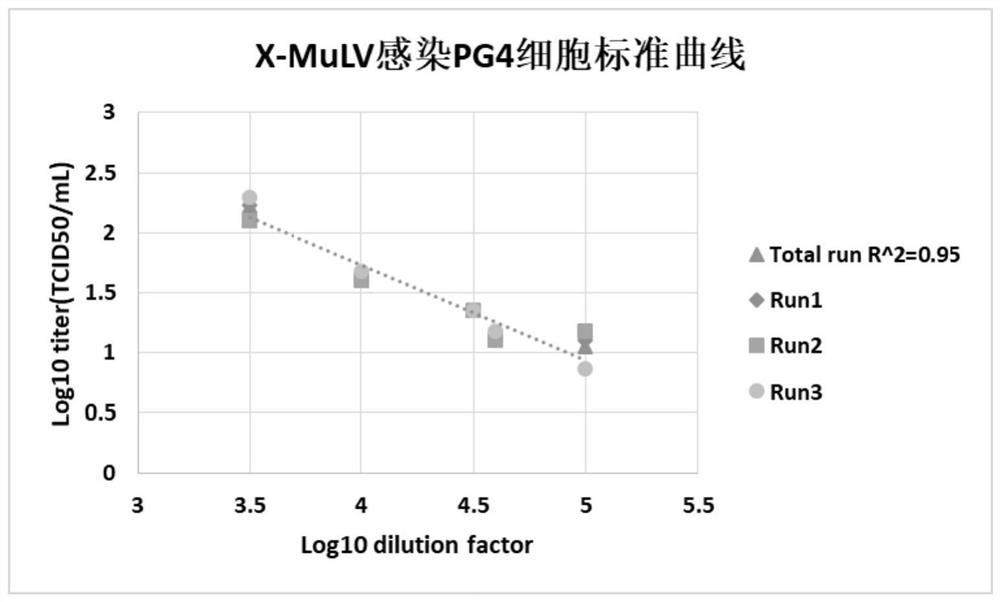
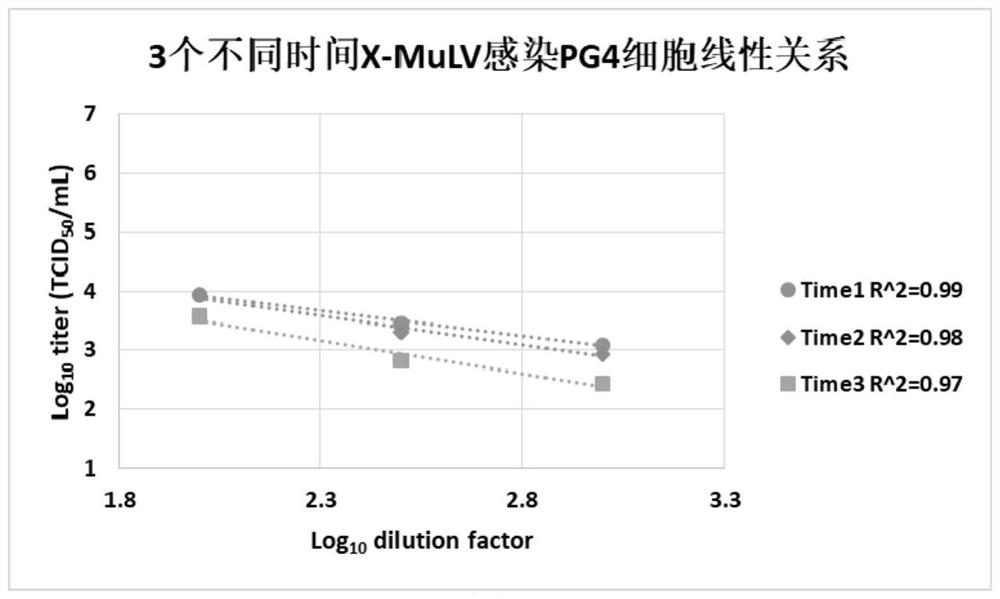
Method for staining and determining titer of heterophilic mouse leukemia virus by using tissue half-infection method
PendingCN112359139AOvercome the disadvantage that genetic stability cannot be guaranteedReduce subjective errorMicrobiological testing/measurementBiological material analysisCytopathic effectStaining
Owner:SUZHOU YAOMING KANGDE INSPECTION TESTING
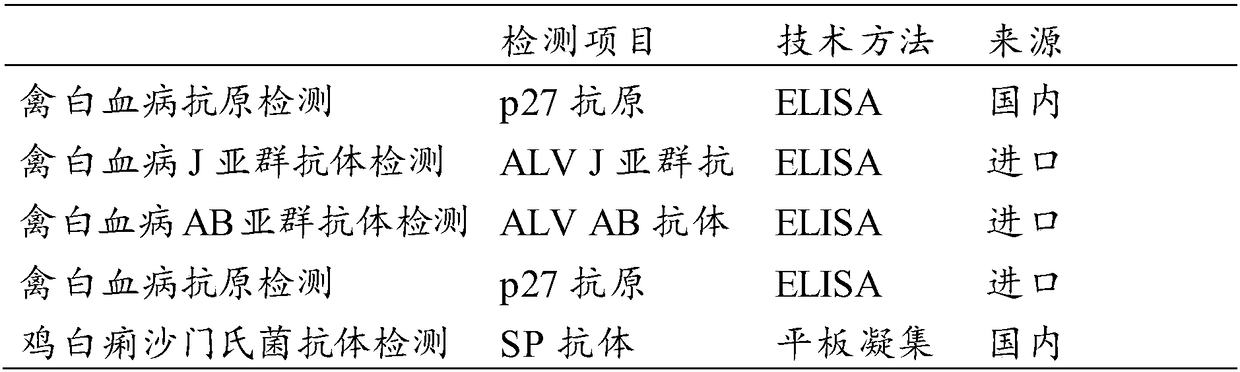
Kit for simultaneously detecting avian leukosis virus antibody and salmonella pullorum antibody
The invention relates to the technical field of biology and in particular relates to a kit for simultaneously detecting an avian leukosis virus antibody and a salmonella pullorum antibody. An antigencombination of the kit comprises p27 protein, gp85 protein and GroEL-delta8-1 protein. Avian leukosis virus capsid protein p27, prokaryotic expression protein of envelope protein gp85, and prokaryoticexpression protein of truncated GroEL-delta8-1 of a salmonella pullorum dominant antigen are used as a coating antigen to develop an ELISA (Enzyme-linked Immunosorbent Assay) kit capable of simultaneously detecting the avian leukosis virus antibody and the salmonella pullorum antibody. Compared with a current common kit for independently detecting avian leukosis or pullorum disease, the kit provided by the invention has the effect of simultaneously detecting the avian leukosis or the pullorum disease, and the detection and purification work of the avian leukosis and the pullorum disease can be extremely alleviated.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com