Novel peptide, preparation method thereof, pharmaceutical composition comprising novel peptide and application of novel peptide
A composition and drug technology, applied in the field of active peptides, can solve the problems of cumbersome purification methods and achieve the effects of simple separation methods, strong analgesic effect and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The following examples are used to further illustrate the present invention, but this does not imply any limitation to the present invention. Embodiment 1 Active peptide separation and purification of the present invention
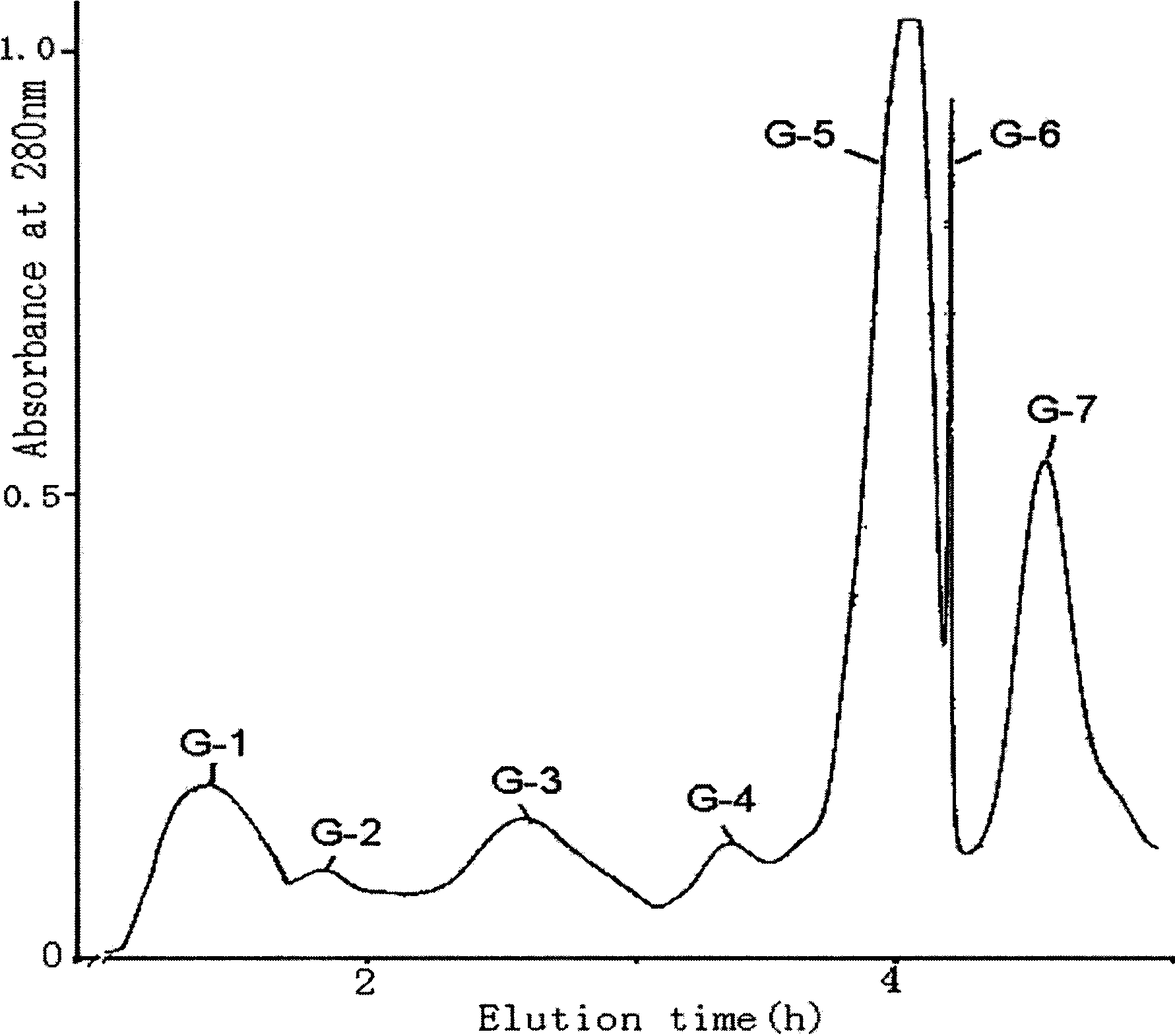
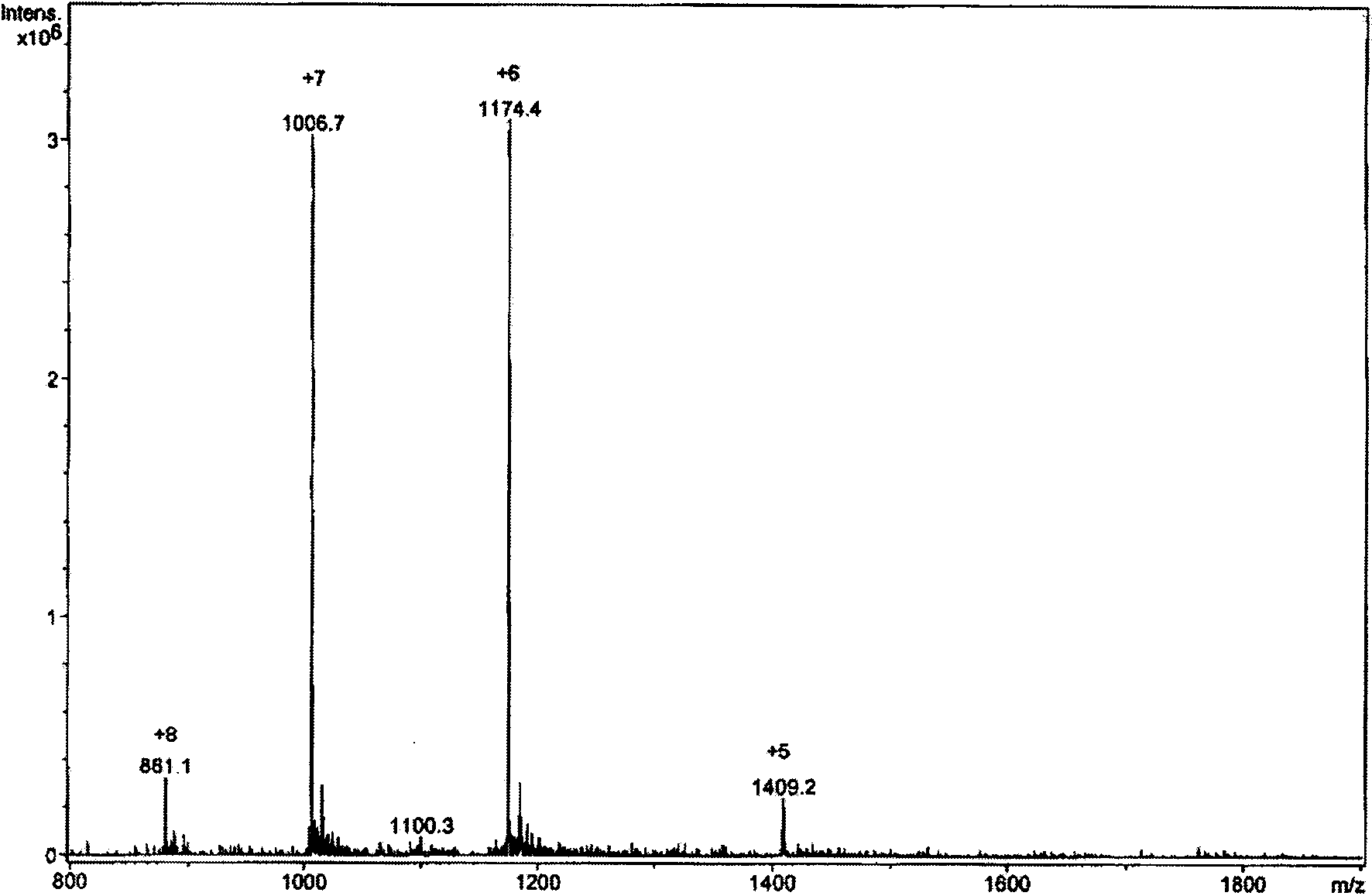
[0024] The crude venom of East Asian scorpion was dissolved in distilled water (1 g was dissolved in 30 ml distilled water), centrifuged at 10000 r / min for 10 minutes, the precipitate was discarded, and the supernatant was freeze-dried. Redissolve 500 mg of the lyophilized supernatant in 10 ml of water, separate with Sephadex G-50, elute with water, the column length (2.6×100 cm), collect the analgesic component (about 350 mg), and lyophilize the analgesic component Dissolve in 0.01mol / L PBS (pH6.8), load the sample on the pre-equilibrated CM-Sephadex C-50 ion exchange column (2.6×50cm), first elute with the equilibrium buffer until no protein peak appears, and then Elute with 0.02mol / L NaCL+0.01mol / L PBS. The eluate was collected, dialyzed, and f...
experiment example 1
[0038] The analgesic activity of experimental example 1 active peptide of the present invention is measured
[0039] The analgesic activity was measured using the mouse acetic acid writhing model. 0.9% NaCl was used as the blank control, and morphine was used as the positive control. The mice were randomly divided into 6 groups, 10 in each group, and the active peptide of the present invention and the blank control (0.9% NaCl ) and positive control (0.2mg / kg morphine).
[0040] The result is as follows:
[0041]
[0042]
[0043] Note, *p<0.05, **P<0.01, ***P<0.001, compared with the control group.
experiment example 2
[0044] Experimental example 2: Toxicity determination of East Asian scorpion with low toxicity and analgesic active peptide
[0045] The active peptide of the present invention was dissolved in 0.9% NaCl solution, and the mice were randomly divided into 6 groups, two in each group, with different doses (1mg / kg, 5mg / kg, 10mg / kg, 20mg / kg, 50mg / kg) Active peptide of the present invention and blank control (0.9% NaCL) were injected intravenously. It was found that under the dose of <20mg / kg, no toxic reaction was observed, only in the 50mg / kg dose group, the mice had a toxic reaction in 5 minutes, but recovered in 10 minutes. It shows that the toxicity of the active peptide of the present invention is very low.
[0046] Preparation of the pharmaceutical composition:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com