New Gelsmium elegans Benth. alkaloid compound as well as preparation method and application thereof
A technology of gynecine alkaloids and compounds, applied in the field of medicine, achieves the effect of therapeutic index 37, high yield and shortened separation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of formula (I) compound
[0036] 1. Sample: total alkaloids of Gelcyonium, refer to the classic extraction method of Gelkins alkaloids [Chen Zhongliang. Extraction and Separation of Gelkins Alkaloids, Bulletin of Traditional Chinese Medicine, 1987, 12 (5): 41; Xu Jian, Liang Chongzhen. Comparison of extraction methods of total alkaloids of Gelkins, Pharmaceutical Bulletin, 1988, 23 (6): 341-342; Chen Jingfeng, Yuan Hui. Extraction, separation, identification and general toxicity of total alkaloids of Gelkins, Journal of Hunan Agricultural University (Natural Science Edition), 2003, 29 (5): 422-425] obtained from the rhizome of Ficus chinensis, and the extract was vacuum-dried to obtain the crude extract of total alkaloids in brown-red powder form.
[0037] 2. Instrument: TBE-300A high-speed countercurrent chromatograph, Shanghai Tongtian Biotechnology Co., Ltd.
[0038] 3. Method:
Embodiment 2
[0043] Embodiment 2: identification of formula (I) compound
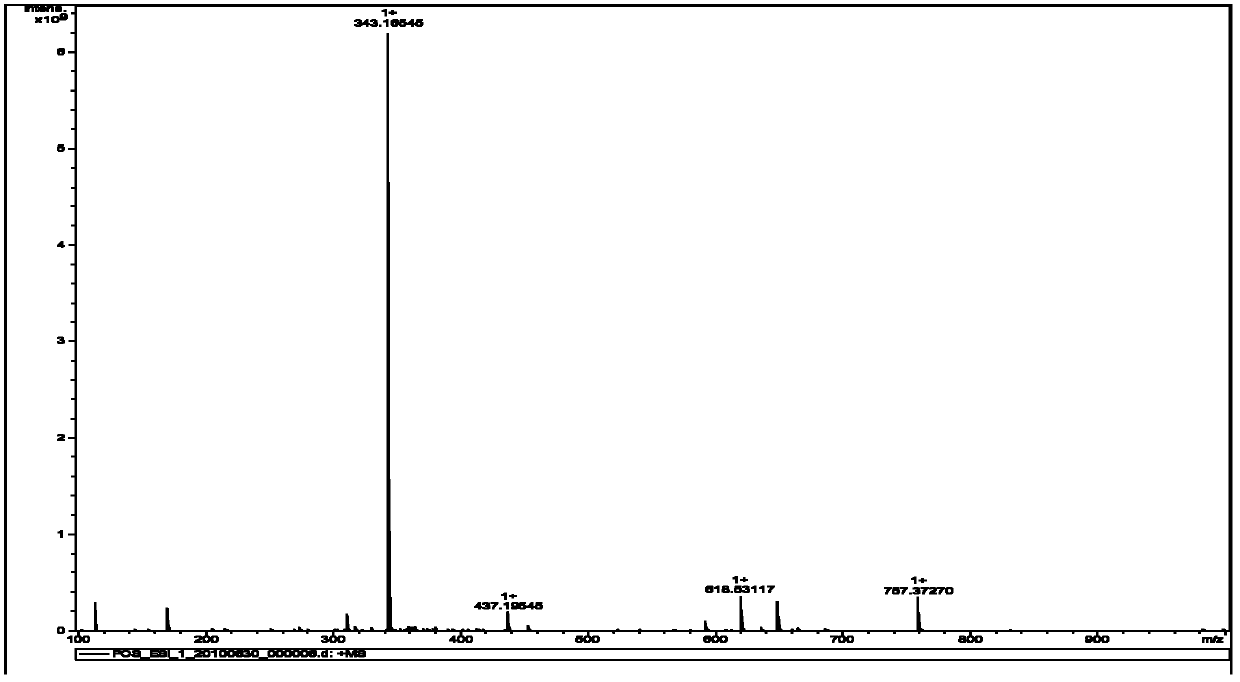
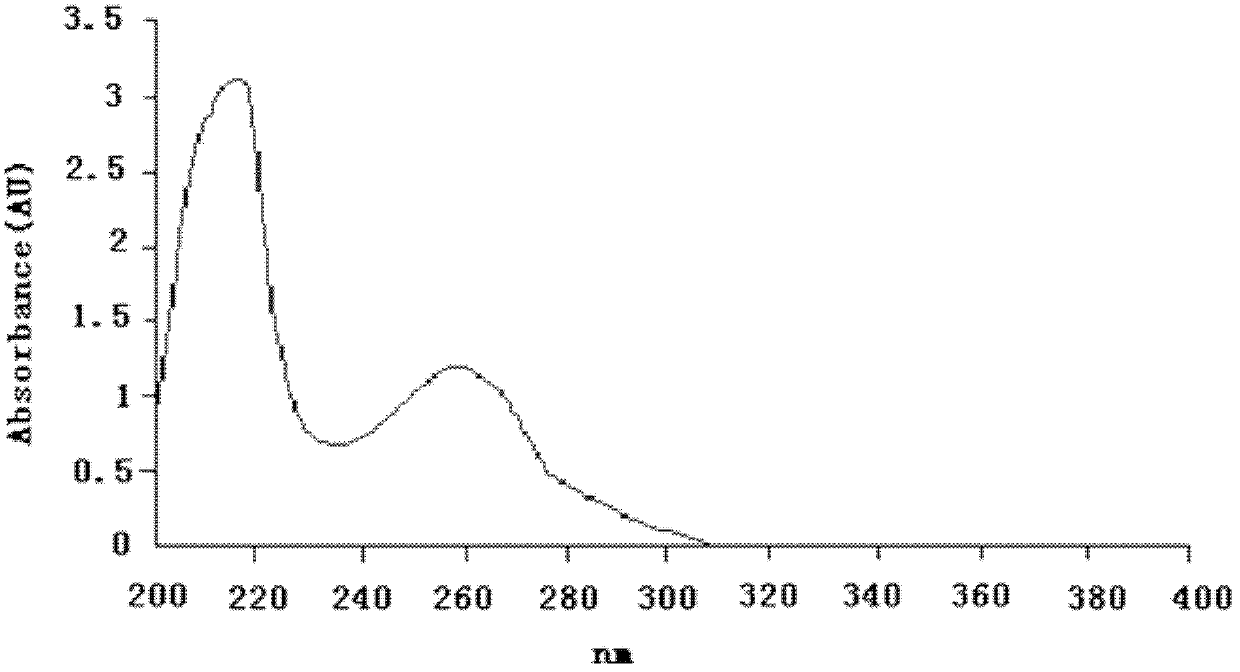
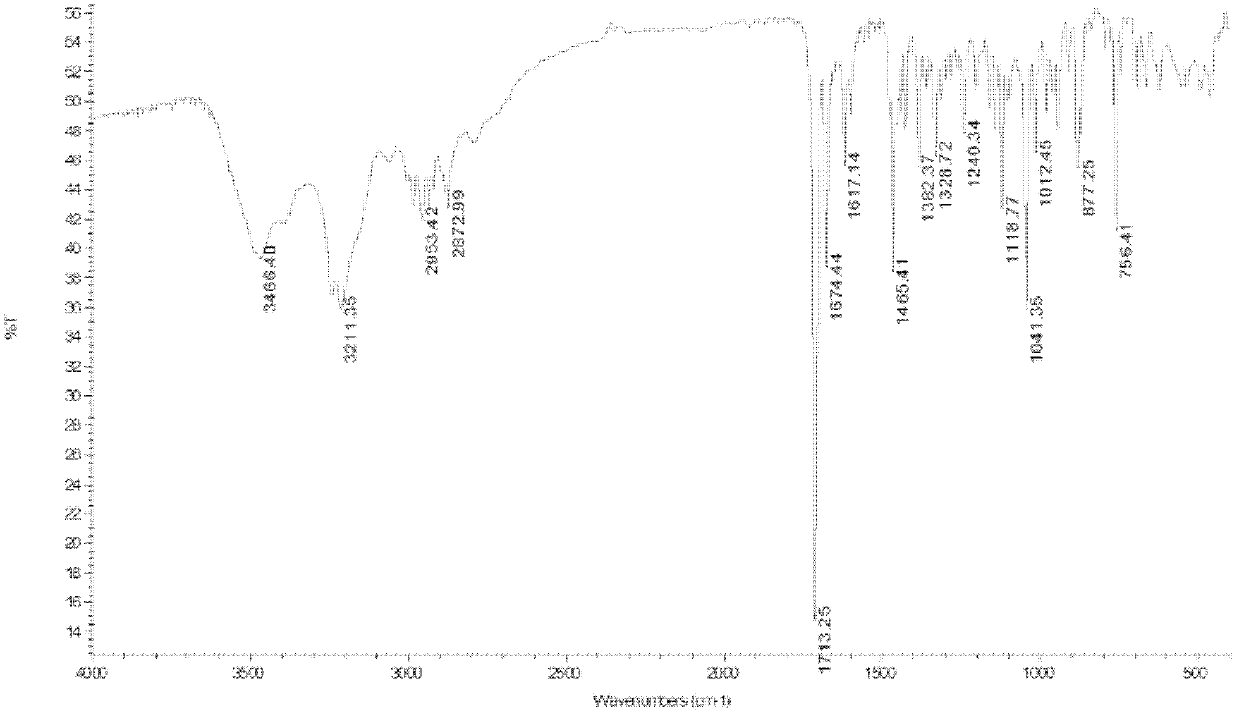
[0044] The target compound of above-mentioned light yellow crystal, in high-resolution mass spectrometry ESI-MS (see figure 1 ) gives the molecular ion peak [M+H] + m / z 343.1655, thus determining the molecular weight to be 342.1655. UV spectrum (see figure 2 ) The maximum absorption peak wavelength λmax (ethanol solvent) is located at 215 nm and 234 nm, showing the characteristic absorption of the indole ring. IR spectrum (see image 3 ) 1713.25cm -1 Shows carbonyl absorption, 1465.41cm -1 、1617.14cm -1 、756.41cm -1 Shows benzene ring absorption, 2850-2960cm -1 For methyl and methylene absorption, 1300-1000cm -1 It is absorbed by ether bond. 1 H-NMR (CDCl 3 and CD 3 OD) (see Figure 4 ) shows four groups of aromatic hydrogen signals δ7.62 (1H, d, 9-H, J =7.6 Hz), 7.20 (1H, td, 10-H, J =7.6, 1.0 Hz), 7.41 (1H, td, 11-H, J =7.8, 1.0 Hz), 7.06 (1H, d, 12-H, J =7.7 Hz); a methoxy signal δ3.99 (3H...
Embodiment 3
[0047] Embodiment 3: the acute toxicity test of formula (I) compound
[0048] 1. Drugs and reagents:
[0049] The compound of formula (I) was prepared by the above method with a purity of 98.3%. The compound of formula (I) was accurately weighed and diluted with normal saline to the required solution for each dosage group below.
[0050] 2. Animals
[0051] Kunming male mice, weighing 18-22 g, clean grade, provided by Shanghai Slack Experimental Animal Co., Ltd. The room temperature is 20-25°C, food and drink are free, and the light-dark cycle is 12 / 12 h. It is used for formal experiments after 3 days of adaptation to the laboratory.
[0052] 3. Method
[0053] The mice were randomly divided into 6 groups, 10 in each group, each group of mice were subcutaneously injected with 0.7 mg / kg, 0.49 mg / kg, 0.343 mg / kg, 0.24 mg / kg, 0.168 mg / kg, 0.118 mg / kg The compound of formula (I) was observed for 7 days and the death of mice was recorded, and the LD of the compound was calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com