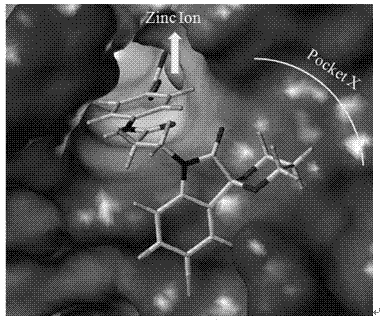
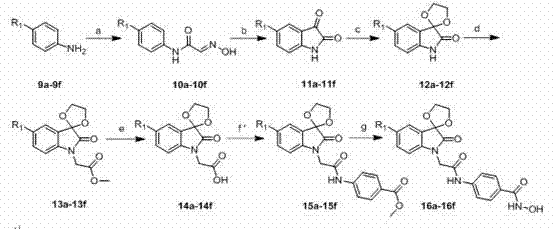
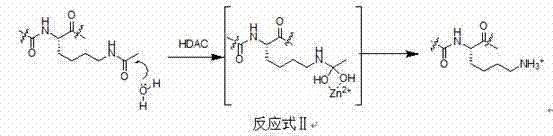
Isatin histone deacetylase inhibitors as well as preparation method and applications thereof
A histone and inhibitor technology, applied in anti-inflammatory agents, organic chemistry, antiviral agents, etc., can solve the problems of histone deacetylase inhibitor enzyme inhibitory activity and cell tumor inhibitory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1. Synthesis of compound 8a of the present invention
[0094] (1) 5-Bromoisatin 3
[0095] Add 40mL of water to a three-necked bottle equipped with an electric stirrer, a thermometer and a constant pressure dropper, add anhydrous sodium sulfate (90g) when warming, start the electric stirrer to make it even, add 20ml of chloral hydrate (0.06mol ) aqueous solution, and then slowly drop the solution prepared by p-bromoaniline (1) (0.04mol), concentrated hydrochloric acid (10mL) and water (70mL), with the dropping of p-bromoaniline, a large number of flocculent precipitates will be produced. An aqueous solution (40 mL) of hydroxylamine hydrochloride (0.13 mol) was then added dropwise. After the dropwise addition, the temperature was raised to 85°C, and the reaction progress was monitored by TLC, and the reaction was stopped after 1.5-2.5 hours. The reaction system was rapidly cooled to room temperature, filtered with suction, and dried in the air to obtain the c...
Embodiment 2
[0105] Example 2. Synthesis of compound 8b of the present invention
[0106] The preparation method is the same as that of compound 8a, except that methyl glycine in the reaction reagent is replaced by methyl 4-aminobutyrate. 1H NMR (600 MHz, DMSO) δ 10.36 (s, 1H), 8.70 (s, 1H), 8.27 (t, J = 5.5 Hz, 1H), 7.60 (dd, J = 6.8, 2.0 Hz, 2H), 6.90 – 6.87 (m, 1H), 4.33 (dd, J = 4.1, 2.7 Hz, 2H), 4.32 – 4.30 (m, 2H), 4.25 (s, 2H), 3.05 (dd, J = 12.9, 6.7 Hz, 2H ), 1.96 (t, J = 7.5 Hz, 2H), 1.66 – 1.60 (m, 2H).
Embodiment 3
[0107] Example 3. Compounds of the invention 8c Synthesis
[0108] The preparation method is the same as that of compound 8a, except that the glycine methyl ester in the reaction reagent is replaced by 6-aminocaproic acid methyl ester. 1 H NMR (600 MHz, DMSO) δ 10.34 (s, 1H), 8.67 (s, 1H), 8.23 (t, J = 5.5 Hz, 1H), 7.62 – 7.58 (m, 2H), 6.87 (d, J = 8.3 Hz, 1H), 4.34 – 4.32 (m, 2H), 4.32 – 4.30 (m, 2H), 4.24 (s, 2H), 3.05 (dd, J = 12.7, 6.7 Hz, 2H), 1.94 (t, J = 7.4 Hz, 2H), 1.51 – 1.44 (m, 2H), 1.40 (dt, J = 14.6, 7.1 Hz, 2H), 1.27 – 1.20 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com