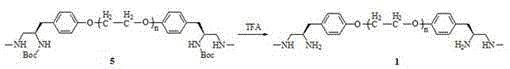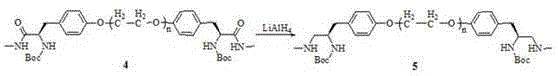Polyethylene glycol-supported bis(S)-2-(4'-benzyloxy)-N-methyl ethane-1,2-diamine and preparation method and application thereof
A polyethylene glycol, methyl ethane technology, applied in the preparation of carboxylates, chemical instruments and methods, preparation of organic compounds, etc. Symmetric catalytic reaction is difficult to achieve large-scale industrial application and other problems, to achieve the effect of easy online detection, high stereoselectivity, and fast reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
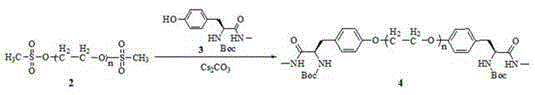
[0028] Example 1-4: Preparation method of polyethylene glycol-supported bis(S)-2-(4'-oxybenzyl)-N-methylethane-1,2-diamine.
[0029]
Embodiment 1
[0031] dry PEG 3400 (10.0 g, 5.8 mmol-OH) was dissolved in dry dichloromethane (100 mL), triethylamine (4.8 mL, 34.8 mmol) was added, and methylsulfonyl chloride was added dropwise at 0°C at 1 drop / second (1.4 mL, 17.4 mmol), react at 40°C for 6 h. The reaction solution was cooled to room temperature, washed with saturated NaCl solution (15 mL×3), anhydrous MgSO 4 Dry, filter, concentrate the filtrate, add anhydrous glacial ether (300 mL) to precipitate, filter, wash with glacial ether several times until TLC (ethyl acetate / petroleum ether=1 / 4, volume ratio) detects that there is no small molecule impurity in the product, The compound was dried under vacuum 2 (10.4 g, 99%). IR (NaCl): υ 2887, 1467, 1343, 1280, 1148, 1114, 963, 842 cm -1 ; 1 H NMR (600 MHz, CDCl 3 ): δ 3.77-3.53 (m, (OCH 2 CH 2 ) n ), 3.16-3.11 (m, 4H), 1.42 (t, J = 6.8 Hz, 6H); 13 C NMR (150 MHz, CDCl 3 ): δ 71.4-69.1, 46.1, 8.8.
[0032]
[0033]
Embodiment 2
[0035] compound 2 (10.0 g, 5.6 mmol) was dissolved in N, N-dimethylformamide (80 mL), and the compounds were added sequentially 3 Namely (S)-3-(4'-hydroxyphenyl)-N-methyl-2-(tert-butoxycarbonylamino)propionamide (3.31 g, 11.2mmol), Cs 2 CO 3 (3.67 g, 11.2 mmol) and 18-crown-6 (0.15 g, 0.56 mmol), react at 60°C for 12 h. N, N-dimethylformamide was distilled off under reduced pressure, the residue was dissolved in dichloromethane (100 mL), and the insoluble matter was filtered off. The filtrate was washed with saturated NaCl solution (15 mL×3), anhydrous MgSO 4 Dry, filter, concentrate the filtrate, add anhydrous glacial ether (300 mL) to precipitate, filter, wash with glacial ether several times until TLC (ethyl acetate / petroleum ether=1 / 4, volume ratio) detects that there is no small molecule impurity in the product, The compound was dried under vacuum 4 (10.0 g, 90 %). IR (NaCl): υ 3384, 3359, 2886, 1672, 1650, 1343, 1280, 1148, 1113, 963, 842 cm -1 ; 1 H NMR (400 M...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap