Preparation method of kit for early-to-mid rapid diagnosis of ovarian cancer
A technology for rapid diagnosis of ovarian cancer, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of low sensitivity and accuracy, lower patient survival rate, missed treatment opportunities, etc., and achieve high accuracy and good differential diagnosis , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] The following is a detailed description of the rapid diagnosis kit for early and mid-stage ovarian cancer of the present invention in conjunction with the accompanying drawings and specific examples.
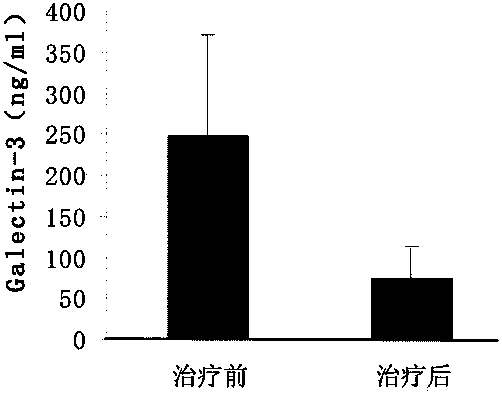
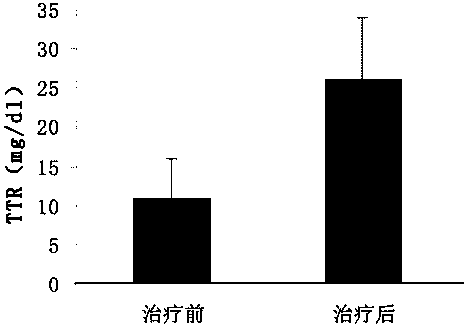
[0058] It is used for early and mid-stage rapid diagnosis kit of ovarian cancer, including capture antibody, capture antibody blocking buffer, standard, labeled antibody, washing solution and chromogenic solution, etc. The labeled antibody is HRP-labeled secondary antibody. The capture antibodies include monoclonal antibodies prepared respectively from galectin-3, transthyretin (TTR) and cancer antigen-125 (CA-125); The joint rapid detection of (TTR) and cancer antigen-125 (CA-125) can effectively improve the detection accuracy and sensitivity, and effectively overcome the shortcomings caused by the detection of cancer antigen-125 (CA-125) alone.
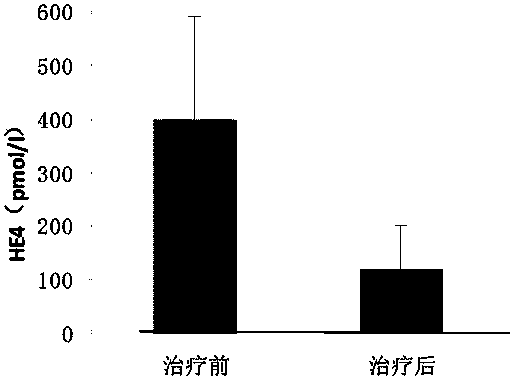
[0059] The capture antibody also includes a monoclonal antibody made with human epididymis protein 4 (HE4), through the dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com