Single-dose pharmaceutical preparation of thyroid hormones t3 and/or t4
A technology for thyroid hormones and pharmaceutical preparations, applied to the pharmaceutical preparations of thyroid hormones T3 and T4, and the field of use in diseases, can solve the problems of increasing the amount of impurities, loss of alcohol solvents, inability to maintain chemical and physical stability, etc., and achieve no problem. The effect of easy contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Formulation and preparation of water-alcoholic solutions
[0083] The formulations are used to prepare doses of up to 200 μg of T4, up to 20 μg of T3, and combinations of the two active ingredients.
[0084] In a typical composition, excipients such as ethanol and glycerol, the concentration of the former may range between 5 and 30% w / v, the latter between 70 and 95% w / v, as required with Use with water.
[0085] A one liter aqueous-alcoholic solution containing 100 g / mL T4 was prepared as follows using the qualitative / quantitative compositions listed below:
[0086] 1) T40.105g
[0087] 2) Ethanol (96%) 243g
[0088] 3) Glycerin (85%) 861g
[0089] The T4 was dissolved in ethanol in a suitable dissolution apparatus under continuous stirring at ambient temperature. When a clear solution with no visible undissolved residue had been obtained, glycerol was added and a homogeneous, clear, colorless solution was obtained with gentle stirring at ambient temperature, th...
Embodiment 2
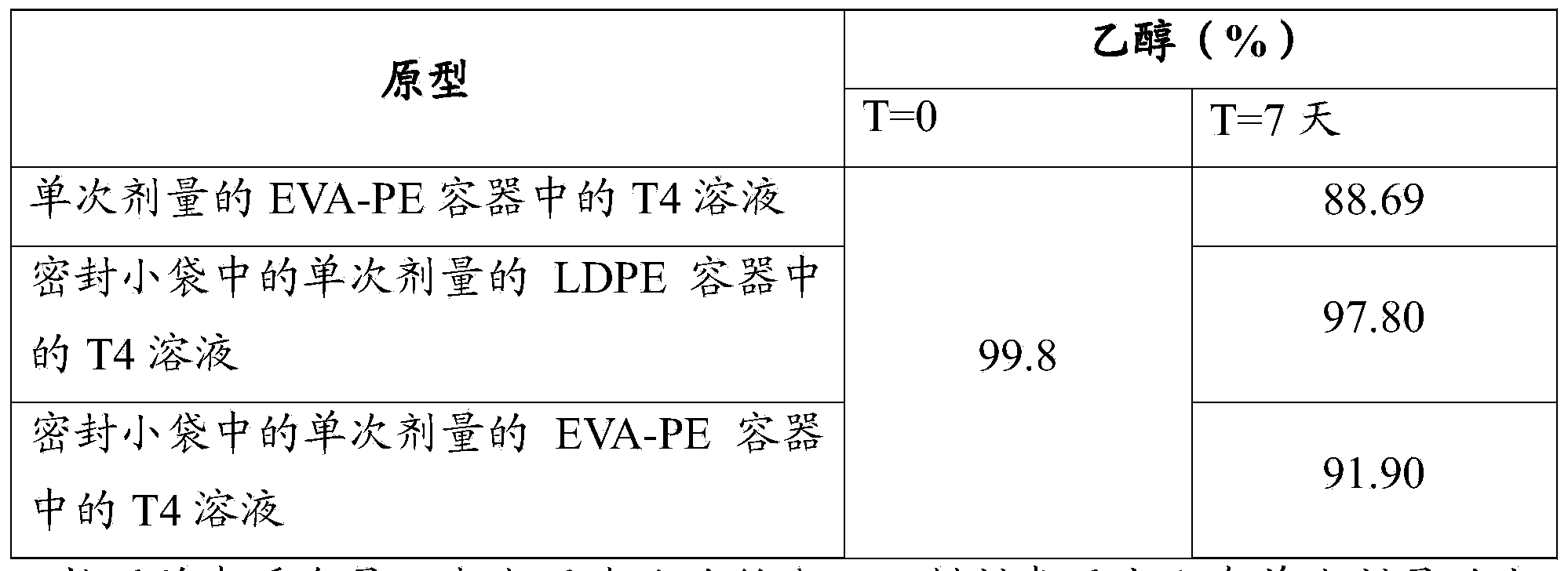
[0091] A solution prepared as described in Example 1, in a nominal volume of 1 mL characterized by different materials, was dispensed between four different one-component single-dose containers:
[0092] A) Squeezable single dose PVC076 container (equivalent to PVC with a thickness of 60 μm);
[0093] B) Squeezable single-dose COPO containers (EMAA copolymer resin-MFI9);
[0094] C) Squeezable single-dose 2 containers (EMAA ionomer resin - some Na&Zn salts and additives) - MFI5;
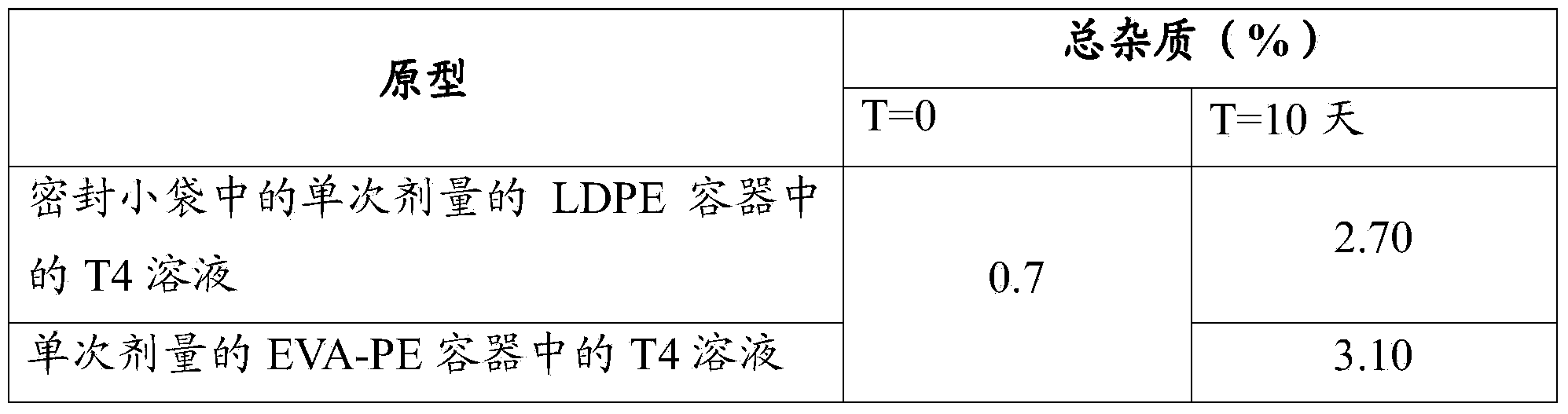
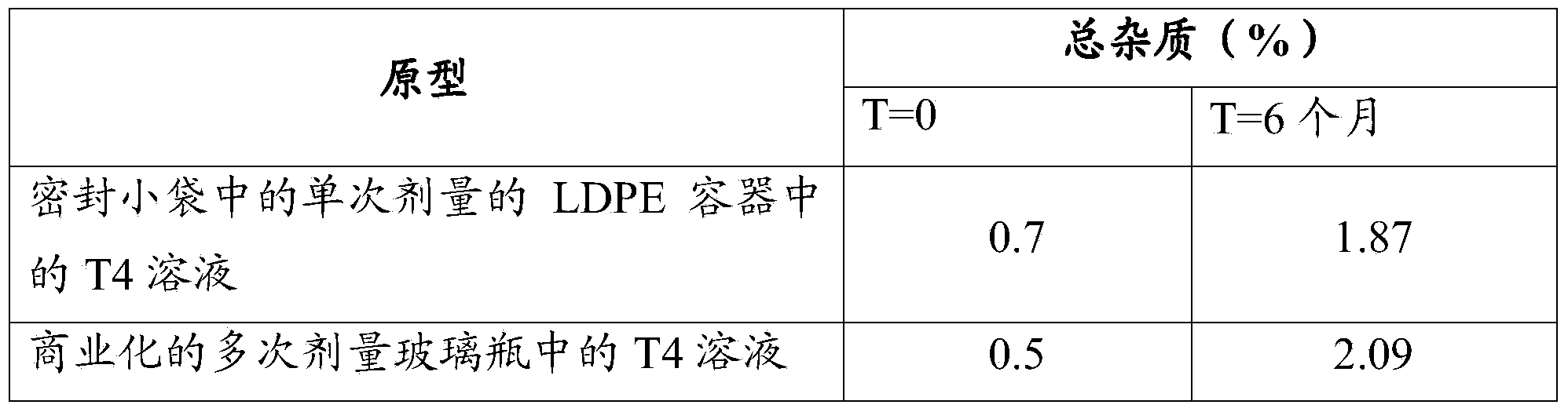
[0095] D) Squeezable single-dose LDPE containers (600 μm thick).
[0096] Melt flow index or melt index (MFI) is the flow index of a molten polymer.
[0097] Filling of single-use containers
[0098] The prototype was prepared on a laboratory scale using an automatic pipette (Gilson P-1000) to fill a disposable container with the aforementioned 1.1 mL glycerol-ethanol solution (prepared as described in Example 1), followed by The containers were sealed with a Pentaseal-lab benchtop sealer.
[...
Embodiment 3
[0104] Single-dose containers made of one-component LDPE plastic with a nominal volume of 1 mL contained in sealed sachets (PET / A1 / PE) were used. Characteristics of the sachet:
[0105] - Layered films with high gas and light barriers: polyester 12 μm, Al 9 μm, polyethylene 50 μm (values to be considered ± 5-6%);
[0106] -Oxygen permeability: 0.1-0.2cc / m2 / day
[0107] -Water vapor permeability: 0.1-0.2g / m2 / day
[0108] The oxygen permeability is measured according to ASTM standard D-3985.
[0109] The water permeability is measured according to ASTM standard E-398.
[0110] Filling of single-use containers
[0111] The prototype was prepared on a laboratory scale using an automatic pipette (Gilson P-1000) to fill a disposable container with the aforementioned 1.1 mL glycerol-ethanol solution (prepared as described in Example 1), followed by The containers were sealed with a Pentaseal-lab benchtop sealer. Pack partial batches in airtight sachets of the type described a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap