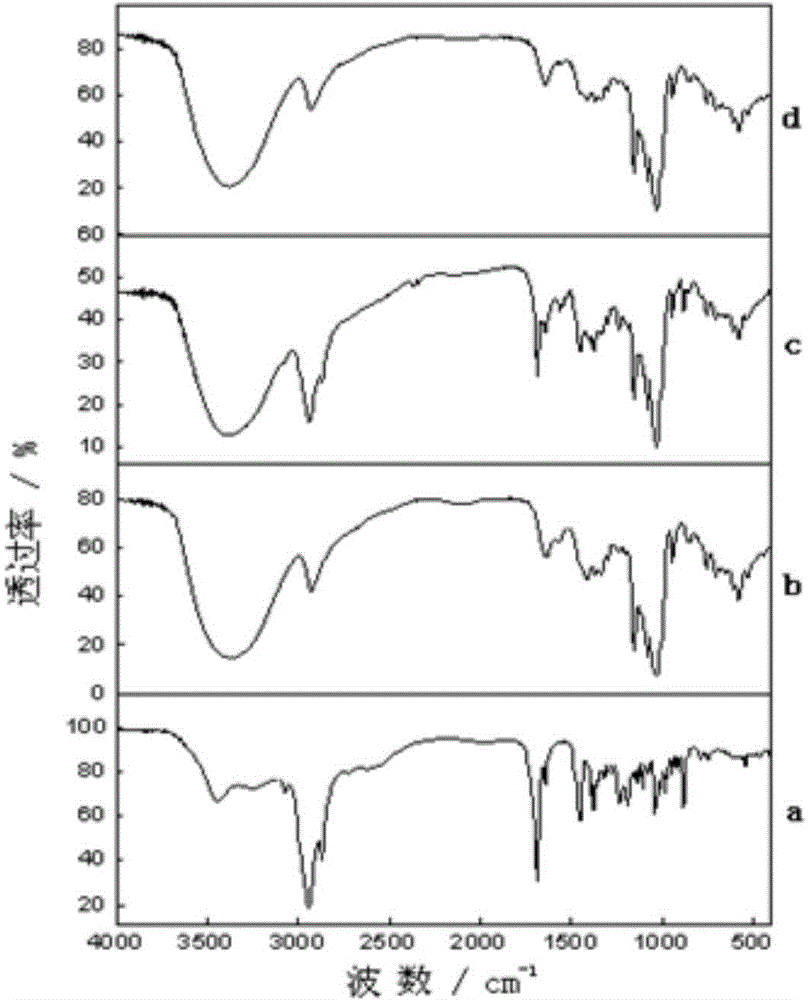
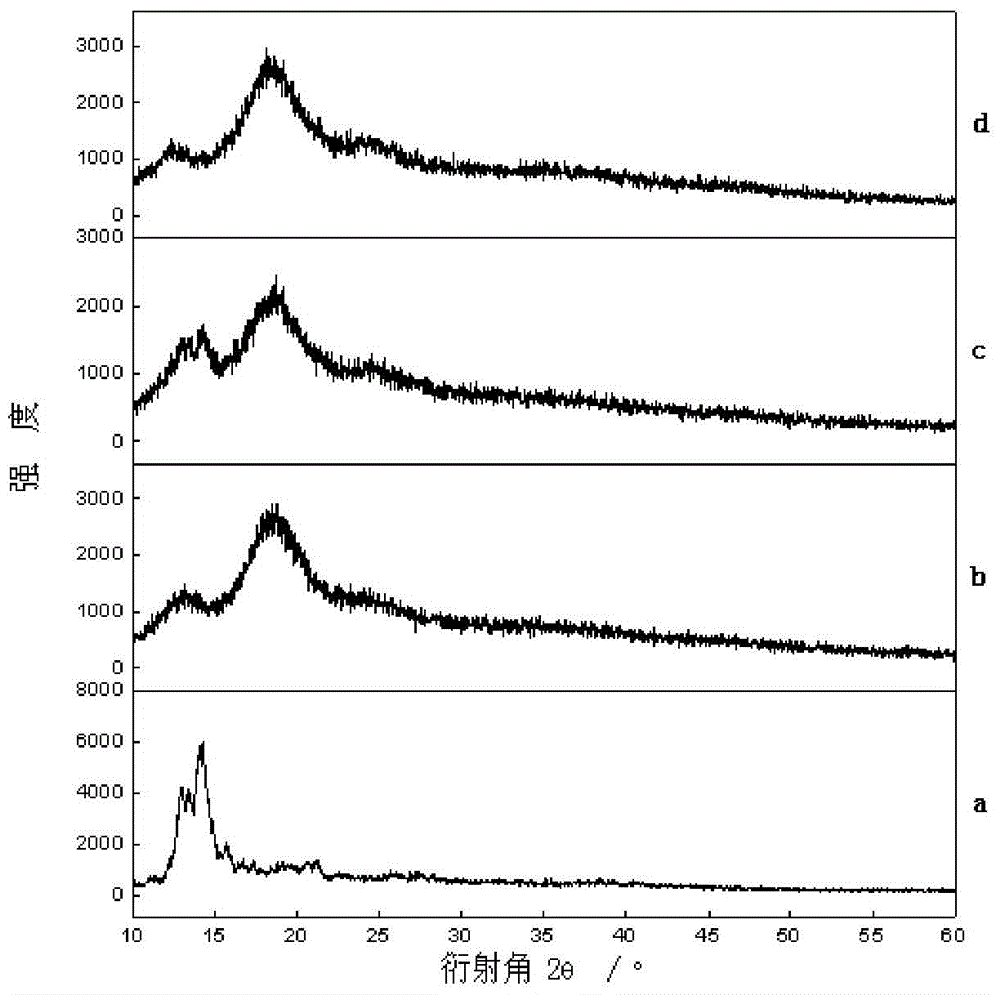
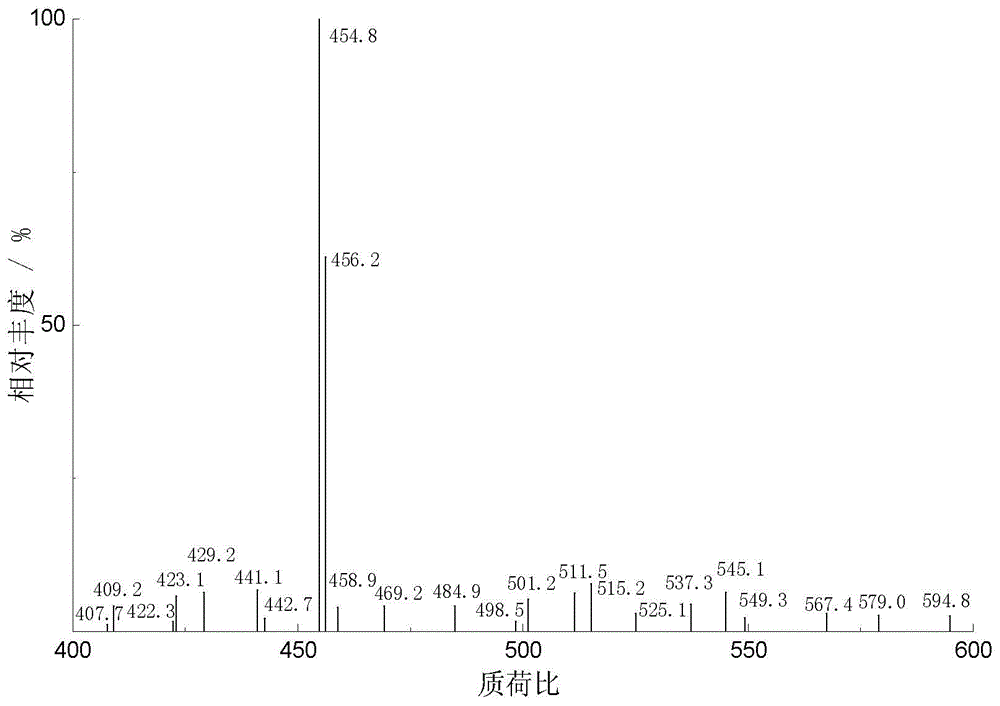
Production method of hydrophilic betulinic acid preparation
A betulinic acid, hydrophilic technology, applied in the directions of anti-inflammatory agents, antiviral agents, pharmaceutical formulations, etc., can solve the problems of low solubility of β-cyclodextrin and low drug inclusion efficiency, and achieve the promotion of inclusion , Improve solubility, increase the effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0017] Specific embodiment one: the preparation method of hydrophilic type betulinic acid preparation of the present embodiment, carry out according to the following steps:
[0018] 1. Weigh 40-60g of β-cyclodextrin and add it to 400-500mL of distilled water to obtain a cyclodextrin solution. Dissolve 6-7g of NaOH in 20-25mL of distilled water to obtain a NaOH solution. Add the prepared NaOH solution dropwise into the cyclodextrin solution to obtain a homogeneous transparent solution, take 7-10g p-toluenesulfonyl chloride and dissolve it in 30-40mL acetonitrile to obtain a mixed solution, add the mixed solution dropwise to the homogeneous transparent solution, and stir at room temperature React for 2 to 4 hours, filter with suction to obtain a precipitate, store the filtrate at 0 to 5°C for 12 hours, and then filter with suction to obtain a precipitate, dry the precipitate obtained twice in vacuum for 12 to 16 hours to obtain a white solid, wash the white solid with absolute et...
specific Embodiment approach 2
[0022] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that in step 2, 2g of 6-p-toluenesulfonyl-β-cyclodextrin, 25 mg of KI and 0.57 mL of diethylenetriamine were dissolved in 5 mL of dry N- in methylpyrrolidone. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0023] Embodiment 3: This embodiment differs from Embodiment 1 or Embodiment 2 in that: the specification of the resin column in step 2 is: 25×450 mm, 724 weakly acidic cation exchange resin, and the resin filling height is 250 mm. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com