Soluble microneedle vaccine patch and preparation method thereof
A soluble, vaccine technology, applied in sheet delivery, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of unsuitable large-scale use, impact, and the microneedle tip is not sharp enough, and achieve sustainable and efficient utilization, The effect of enhancing immune response and enhancing the effect of adjuvant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
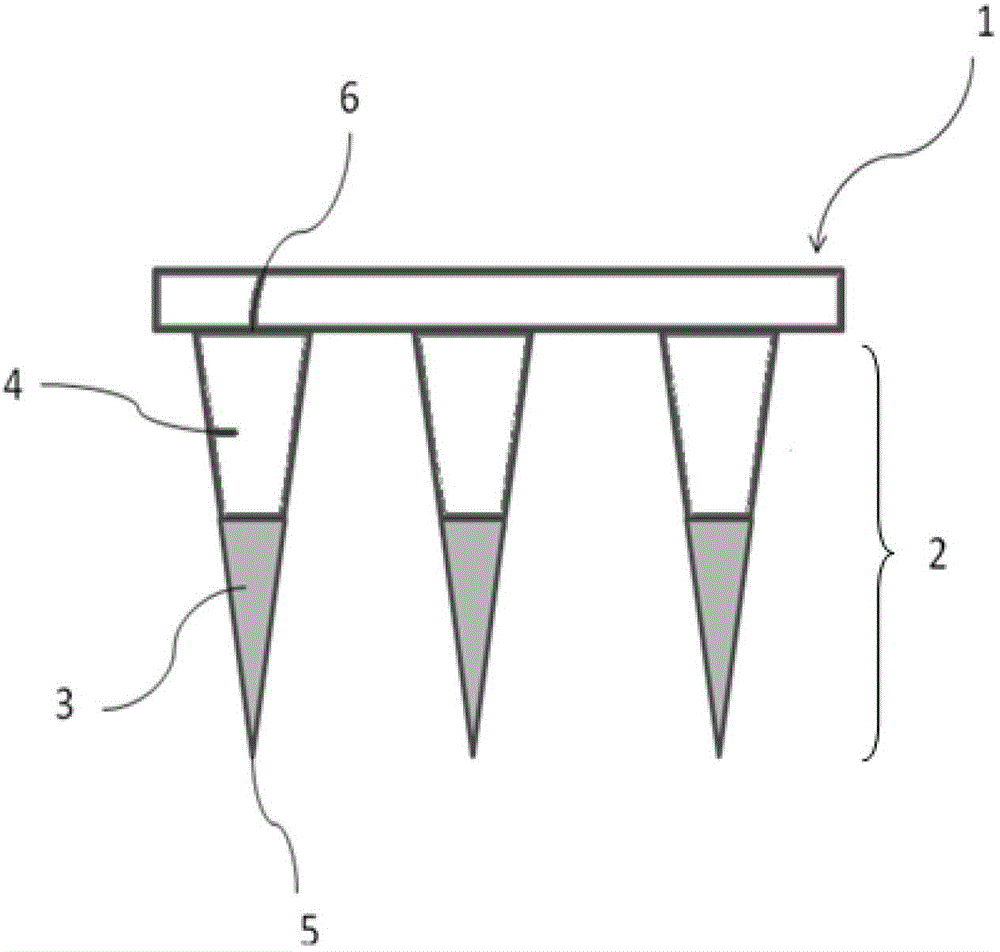
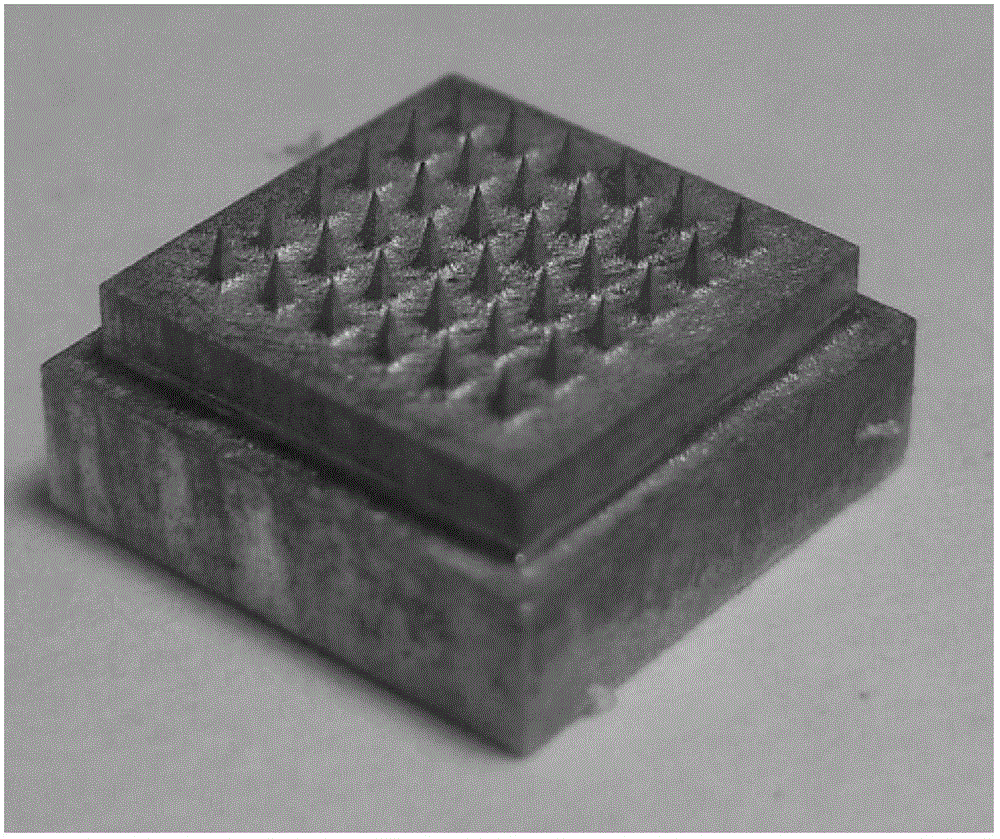
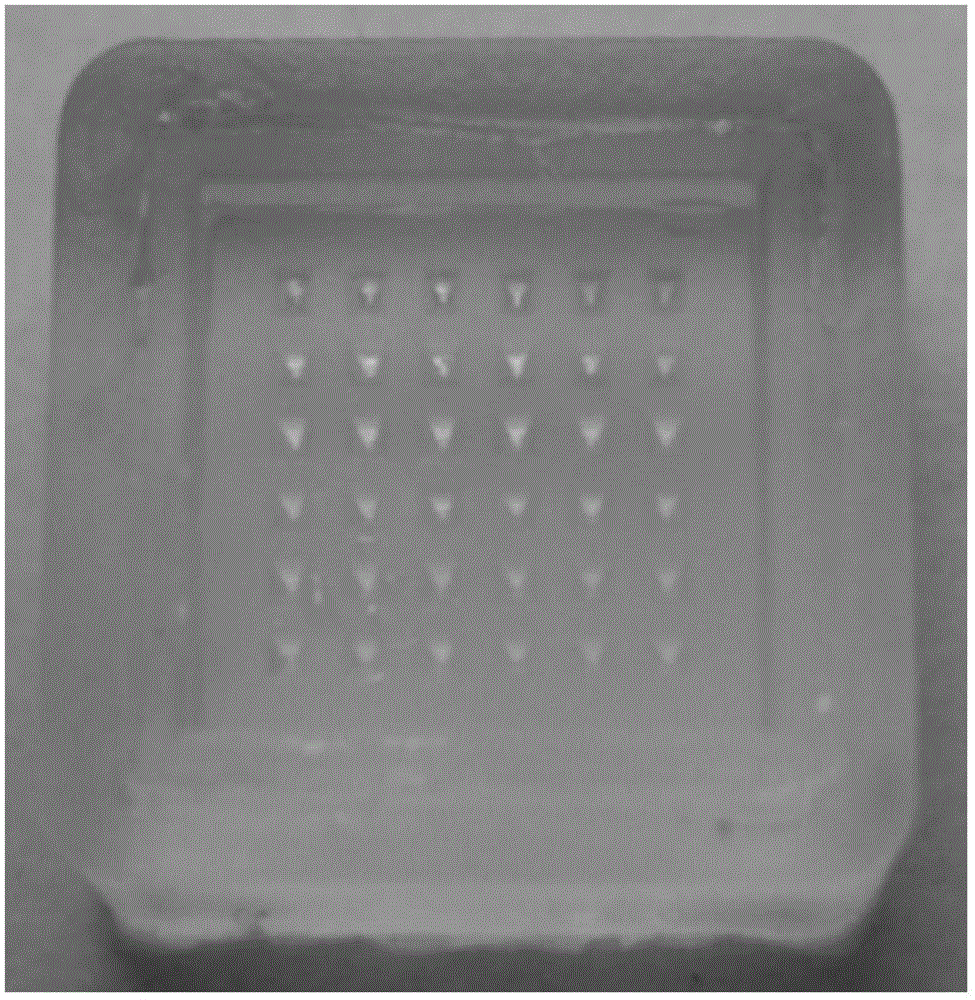
[0064] (1) Preparation of reverse mold: relying on a master mold with 36 (6×6 rows) tetrahedral microneedle bodies with a height of about 650 microns and a bottom diameter of about 250 microns on the surface (such as figure 2 shown), take a mixture of 10 grams of pre-polymerized polydimethylsiloxane and 1 gram of crosslinking agent and cover it on the master mold, and place it at a temperature of 80 ° C for about 30 minutes to obtain a surface with the same The shape and number of microneedle needles on the master mold are suitable for the reverse mold of the required number of pinholes; the surface of the reverse mold contains 36 (6×6 rows) with a depth of about 650 microns and a diameter of the bottom end Pinholes about 250 microns square (such as image 3 shown); the cone angle of the pinhole is 35 degrees;
[0065] (2) Hollow cationic liposome nanoparticles loaded with trypan blue and CpG OND (hollow cationic liposome nanoparticles can be prepared according to the method...
Embodiment 2
[0076] (1) adopt the reverse mold of embodiment 1;
[0077] (2) The hollow cationic liposome nanoparticles loaded with trypan blue and CpG OND (adjuvant) shown in Table 4 (hollow cationic liposome nanoparticles can be prepared according to the method of Comparative Example 3, and The loading method can also be added according to the method of Comparative Example 3) into the 5% HA aqueous solution by weight to obtain three needle preparation solutions respectively, in which the hollow cationic liposomes loaded with trypan blue and CpG OND The weight percentages of the needle body preparation liquid of nanoparticles are 0.1% (the particle diameter of the hollow cationic liposome nanoparticle is 10 nanometers), 20% (the particle diameter of the hollow cationic liposome nanoparticle is 200 nanometers) ), 50% (the particle size of the hollow cationic liposome nanoparticle is 1 micron); then the obtained 3 needle preparation solutions are respectively covered on the 3 surfaces obtai...
Embodiment 3
[0098] A mixture of HBSAg and CpG OND (the weight ratio of HBSAg and CpG OND is 1:2) was substituted for HBSAg in Comparative Example 3, and the drug-loaded hollow cationic nanoparticles described in Comparative Example 3 were used to produce simultaneously loaded Hollow cationic liposome nanoparticles of HBSAg and CpG OND (Lip-HBSAg-CpG OND), the weight ratio of hollow cationic liposome nanoparticles:HBSAg:CpG OND is 5:1:2.
[0099] Add Lip-HBSAg-CpG OND to 5% HA aqueous solution by weight to obtain a needle body preparation solution, wherein the total weight percentage of Lip-HBSAg-CpG OND in the needle body preparation solution is 4% . Except that the above-mentioned needle preparation solution prepared in this example was substituted for the needle preparation solution in Comparative Example 1, the same method and backing preparation solution as in Comparative Example 1 were used to prepare a soluble microneedle vaccine patch. A dissolvable microneedle vaccine patch conta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com