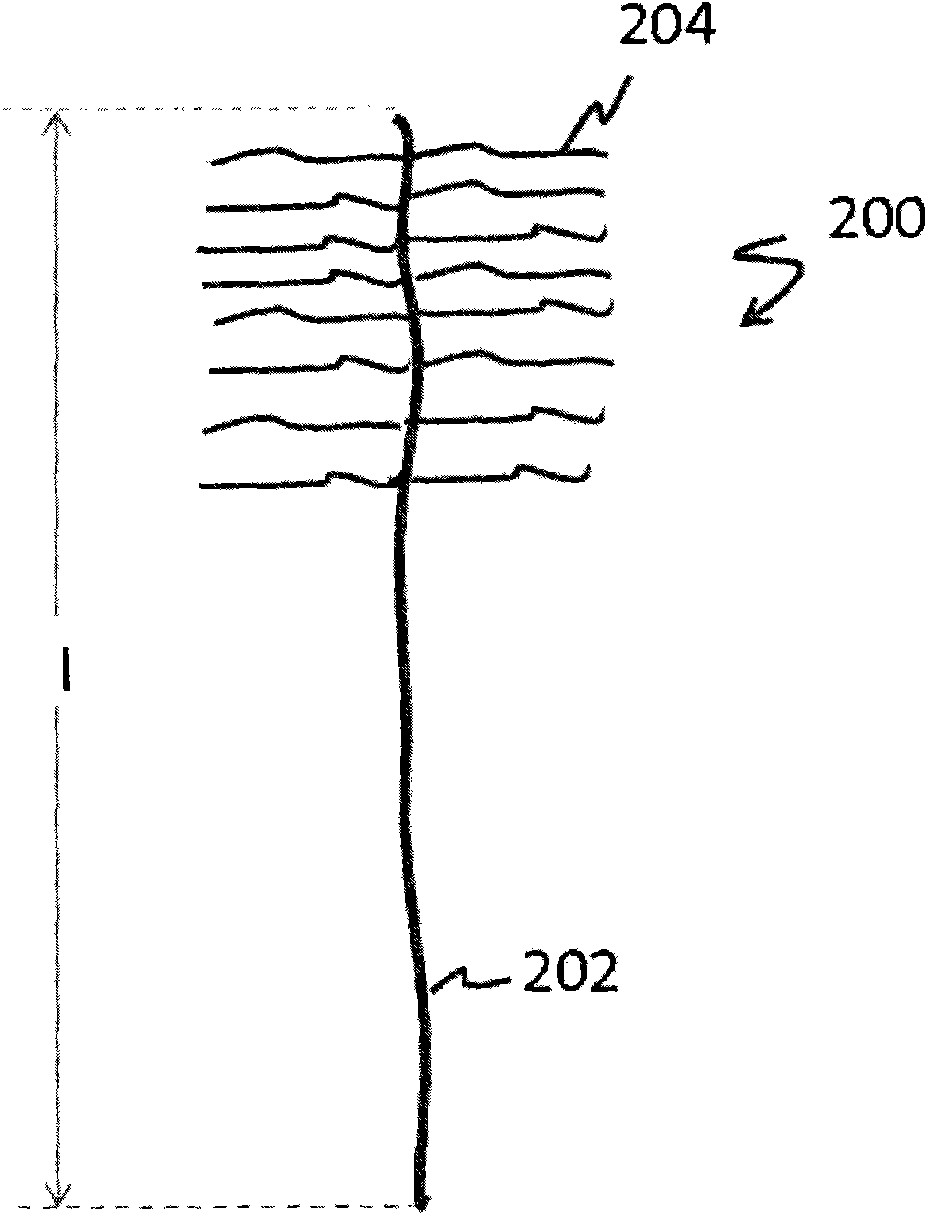
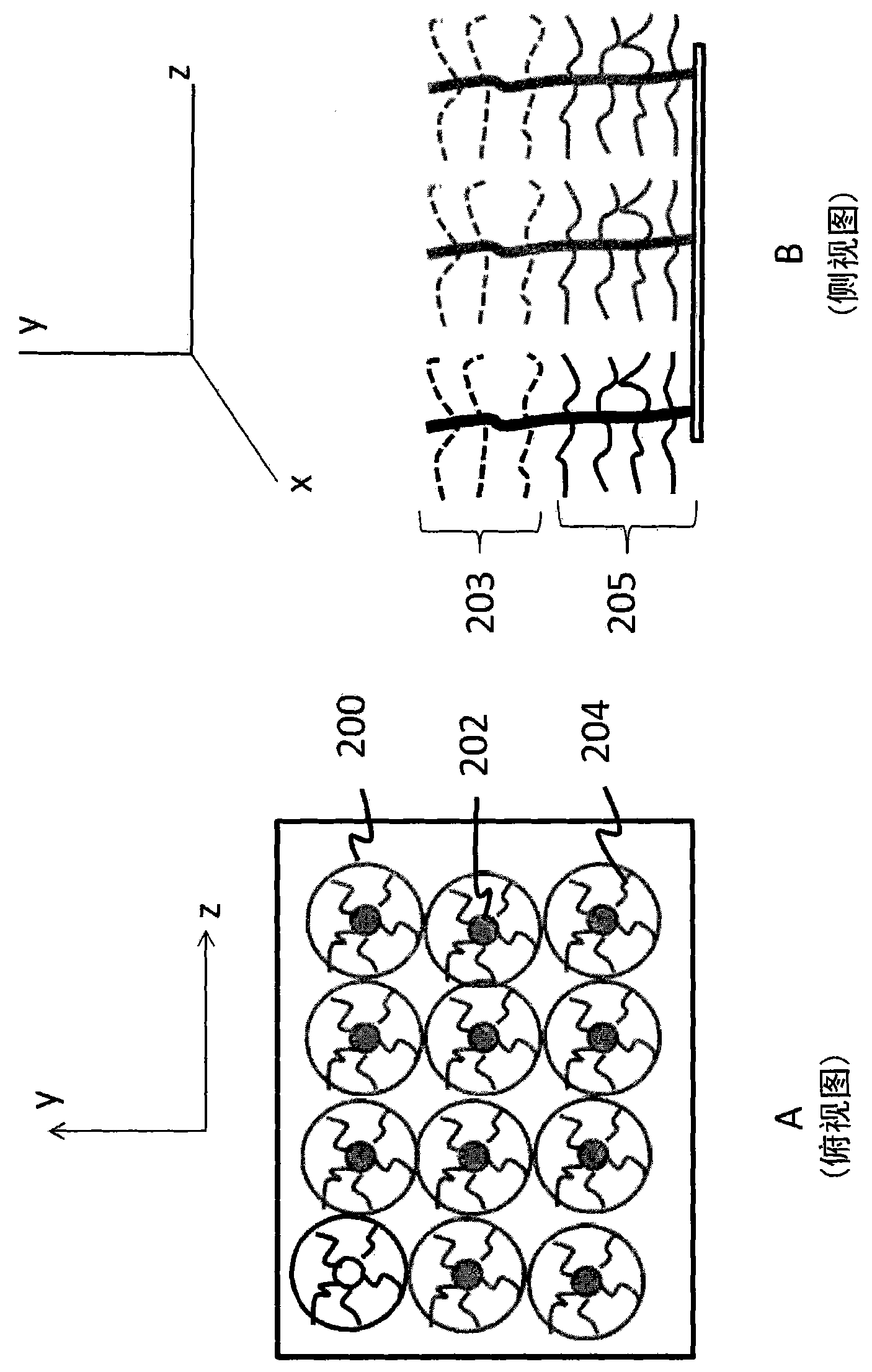
Self-assembled structures, method of manufacture thereof and articles comprising the same
A technology of composition and grafted polymer, which is applied in the photoplate making process of patterned surface, photosensitive materials used in optomechanical equipment, instruments, etc., and can solve the problems of light intensity modulation loss and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] First Polymer-(NB-P(TFpHS) 12 )Synthesis. This example is intended to illustrate the manufacture of the first polymer. The terms used here are as follows: NB-norbornene with chain transfer agent; TF-tetrafluoro; pHS-p-hydroxystyrene; P(TFpHS) 12 ) - poly(tetrafluoro-p-hydroxystyrene) with 12 repeating units.
[0106] The first polymer was produced as follows. Norbornene-chain transfer agent (NB-CTA) (301 milligrams (mg), 0.782 millimoles (mmol)), tetrafluoro-p-hydroxystyrene (TFpHS) (4.49 g, 23.4 mmol), azobis Isobutyronitrile (AIBN) (12.7 mg, 78.2 micromole (μmol)), and 10.5 mL of 2-butanone were added to a 25 mL Schlenk flask equipped with a magnetic stir bar that had been placed under N 2 The atmosphere is dried by flames. The mixture was stirred at room temperature (RT) for 10 minutes (min) and degassed by five freeze-pump-thaw cycles. After the last cycle, the reaction mixture was stirred at RT for 10 minutes and the copolymerization reaction was initiated by...
Embodiment 3
[0112] The second polymer-(NB-P(pHS 13 --PhMI 13 ))Synthesis. This example is intended to illustrate the fabrication of the second polymer. The terms used here are as follows: NB-norbornene with chain transfer agent; pHS-p-hydroxystyrene; PhMI-N-phenylmaleimide; P(pHS13-co-PhMI13)-poly(p- hydroxystyrene-co-N-phenylmaleimide), wherein p-hydroxystyrene is polymerized to contain 13 repeating units and N-phenylmaleimide is polymerized to poly(p-hydroxystyrene ) and also has 13 repeat units.
[0113] The second polymer was produced as follows. NB-CTA (635 mg, 1.65 mmol), pHS (3.95 g, 33.0 mmol), PhMI (5.76 g, 33.0 mmol), AIRN (26.7 mg, 165 μmol), and 35 mL of anhydrous 1,4-dioxane were added into a 100-mL Schlenk flask equipped with a magnetic stir bar that had been placed under N 2 The atmosphere is dried by flames. The mixture was stirred at RT for 10 min and degassed by four freeze-pump-thaw cycles. After the last cycle, the reaction mixture was stirred at RT for 15 minu...
Embodiment 4
[0115] The second polymer-(NB-P(pHS 8 -Co-PhMI 8 ))Synthesis. This example is also intended to illustrate the fabrication of the second polymer. The terms used here are as follows: NB-norbornene with chain transfer agent; pHS-p-hydroxystyrene; PhMI-N-phenylmaleimide; P(pHS 8 -Co-PhMI 8 )-poly(p-hydroxystyrene-co-N-phenylmaleimide), wherein p-hydroxystyrene is polymerized to contain 8 repeating units and N-phenylmaleimide is polymerized to poly (p-hydroxystyrene) and also has 8 repeating units.
[0116] The second polymer was produced as follows. NB-CTA (802 mg, 2.08 mmol), pHS (2.50 g, 20.8 mmol), PhMI (3.60 g, 20.8 mmol), AIBN (16.9 mg, 104 μmol), and 20 mL of anhydrous 1,4-dioxane were added into a 50 mL Schlenk flask equipped with a magnetic stir bar, which had been placed under N 2 The atmosphere is dried by flames. The mixture was stirred at RT for 10 min and degassed by four freeze-pump-thaw cycles. After the last cycle, the reaction mixture was stirred at RT fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com