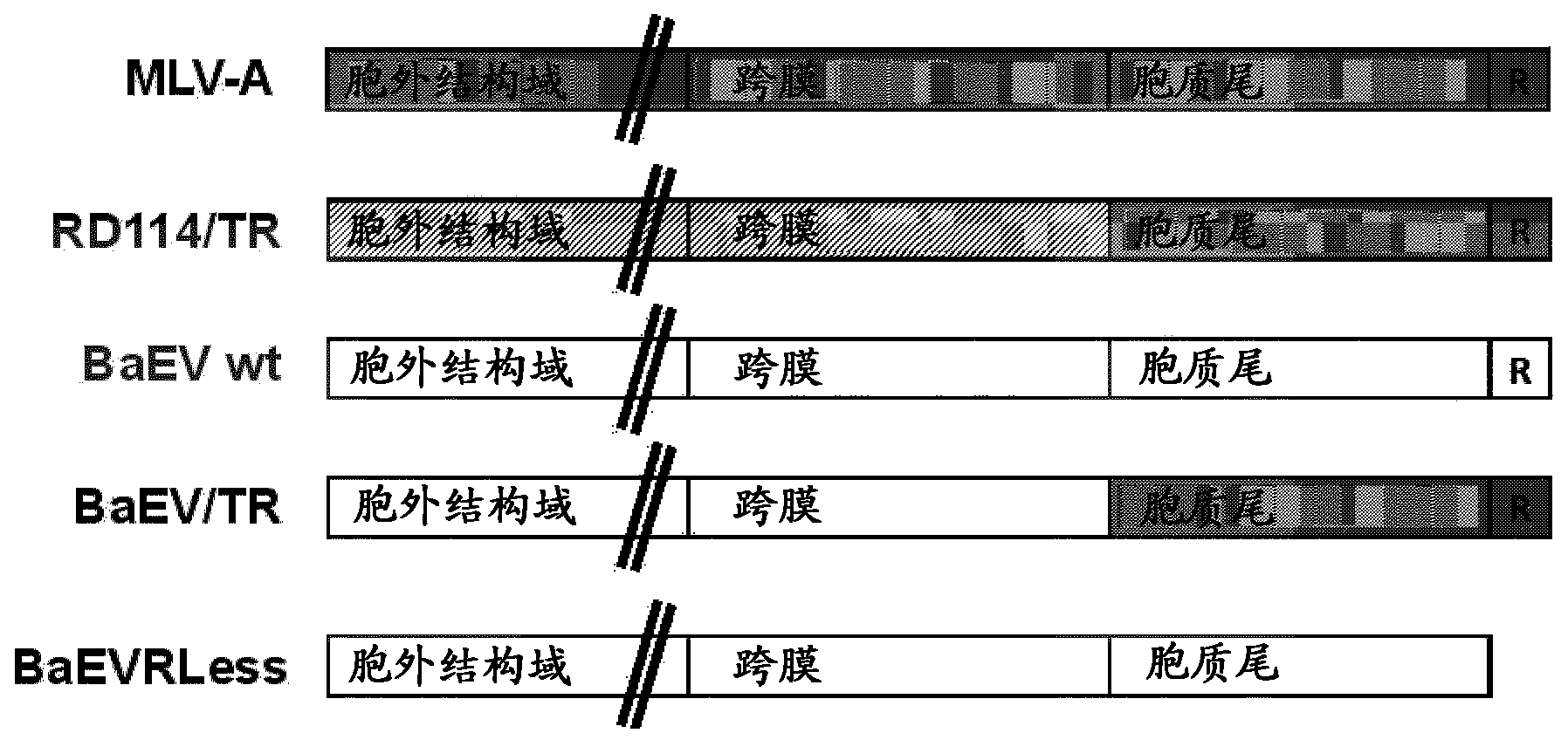
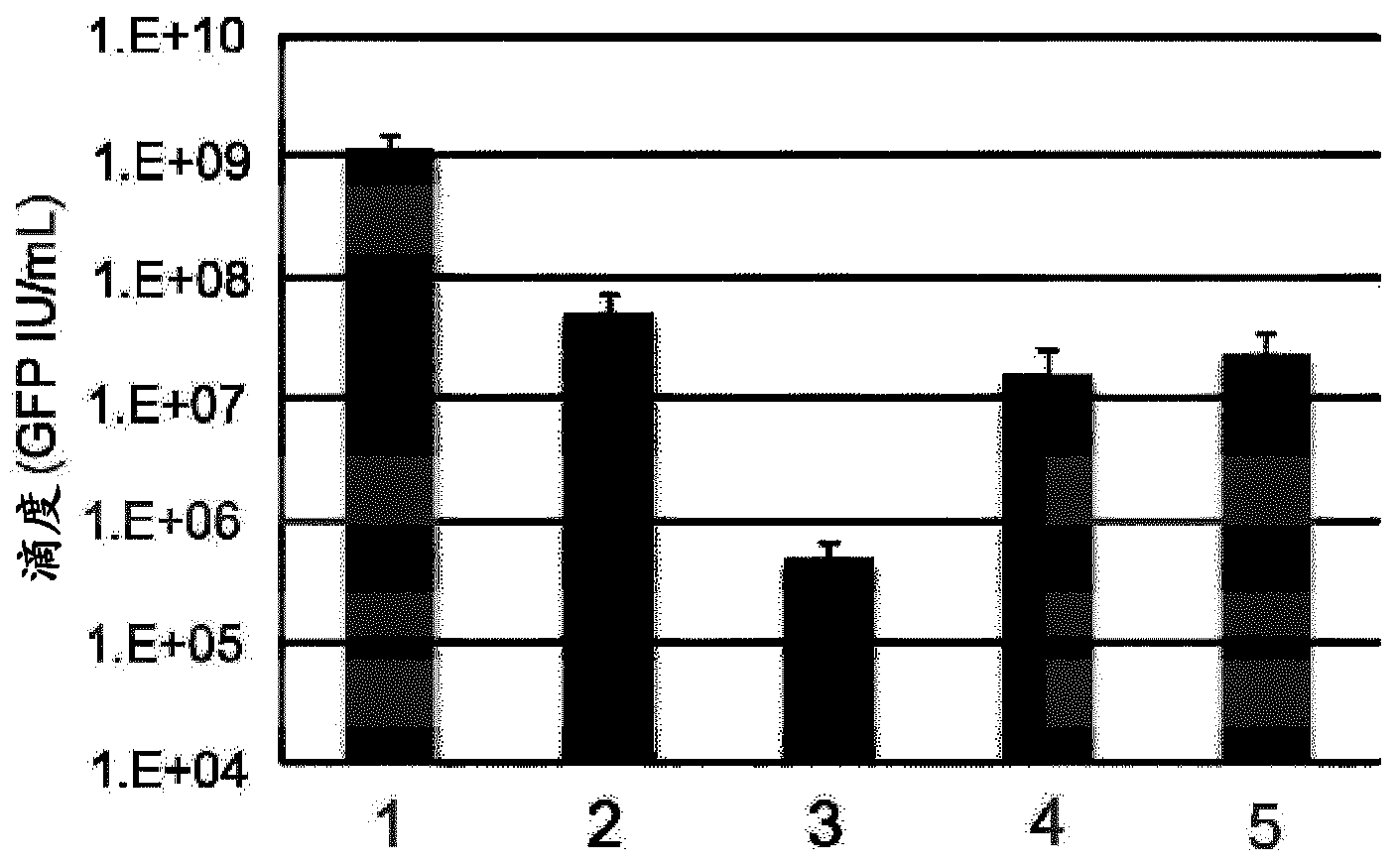
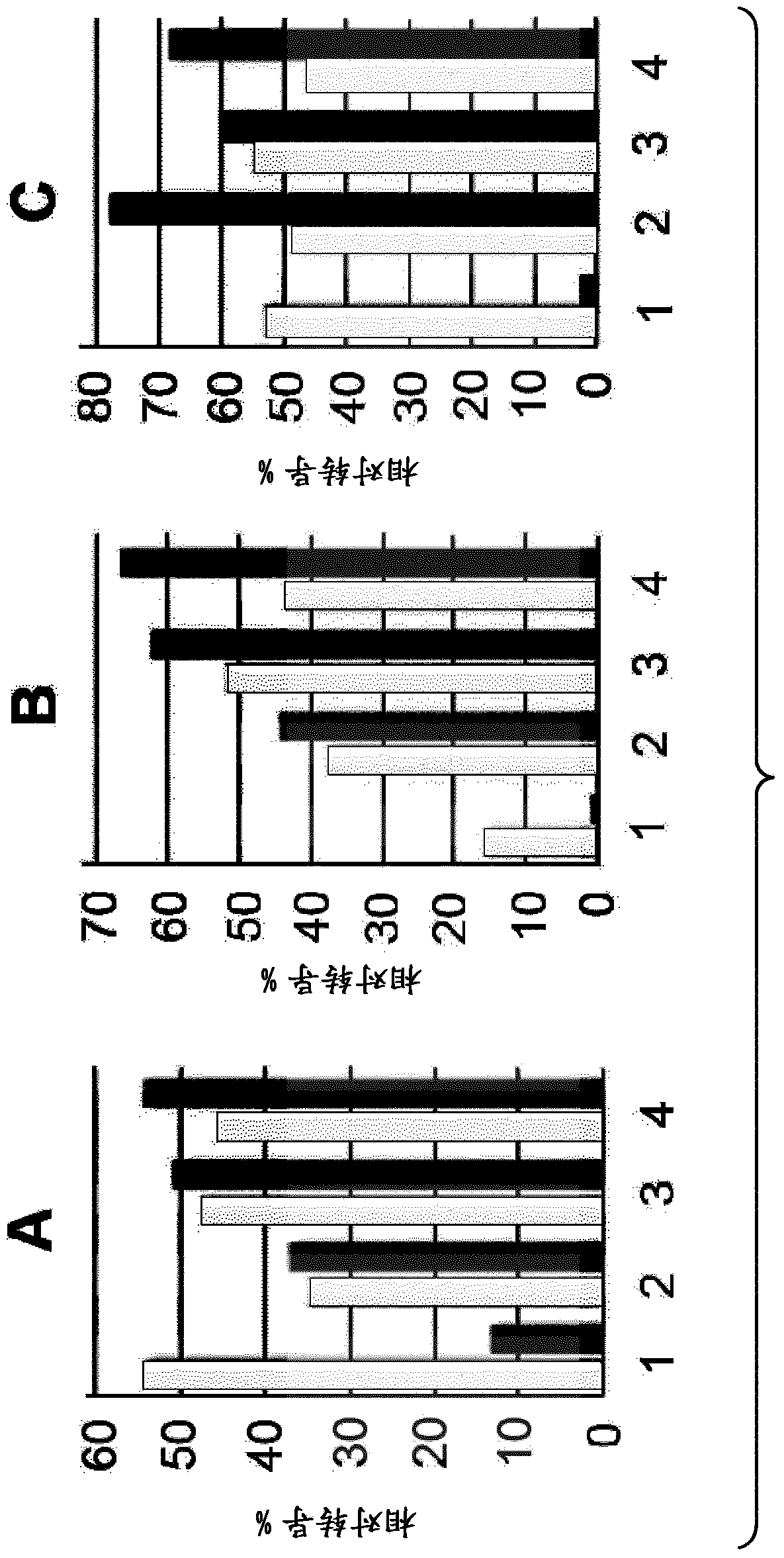
Lentiviral vectors pseudotyped with mutant BaEV glycoproteins
A viral vector, pseudotyping technology, applied in the direction of virus/bacteriophage, introduction of foreign genetic material, virus using vector, etc., can solve problems such as restriction and ineffective entry of glycoproteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0150] The following examples demonstrate the advantageous properties of BaEV / TR- and BaEVRLess pseudotyped lentiviral vector particles for use as gene transfer vectors compared to previous pseudotyped lentiviral vectors.
[0151] Materials and Methods
[0152] Generation and titration of lentiviral vectors
[0153] Self-inactivating HIV-1 -derived vectors were generated by transient transfection of HEK293T cells (American Type Culture Collection, Rockville, MD, CRL-1573). 24 hours before transfection, the 2.610 6 HEK293T cells were seeded to 10-cm 2 on tissue culture dishes. 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal calf serum (FCS). Using 8.6 μg Gag-Pol packaging construct 8.91 and HIV-1 derived SIN transfer vector pHIV-SFFV-GFP-SIN encoding GFP and 2.5 μg pMD.G encoding VSV-G glycoprotein (GP) or 7 μg phCMV-RD114 / Cells were transfected with TR, phCMV-BaEVWT, phCMV-BaEV / TR, or phCMV-BaEVRLess by calcium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com