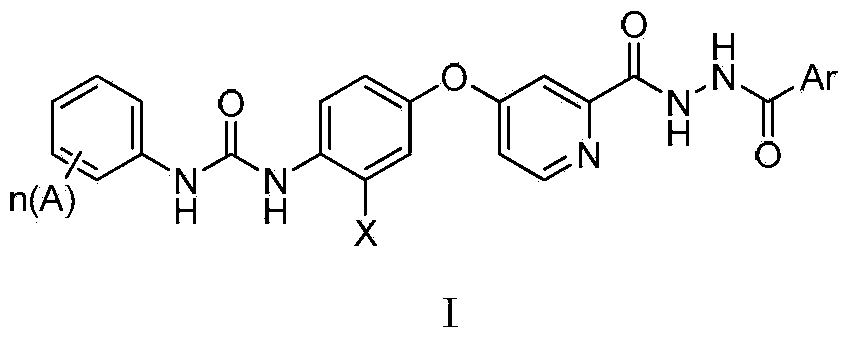
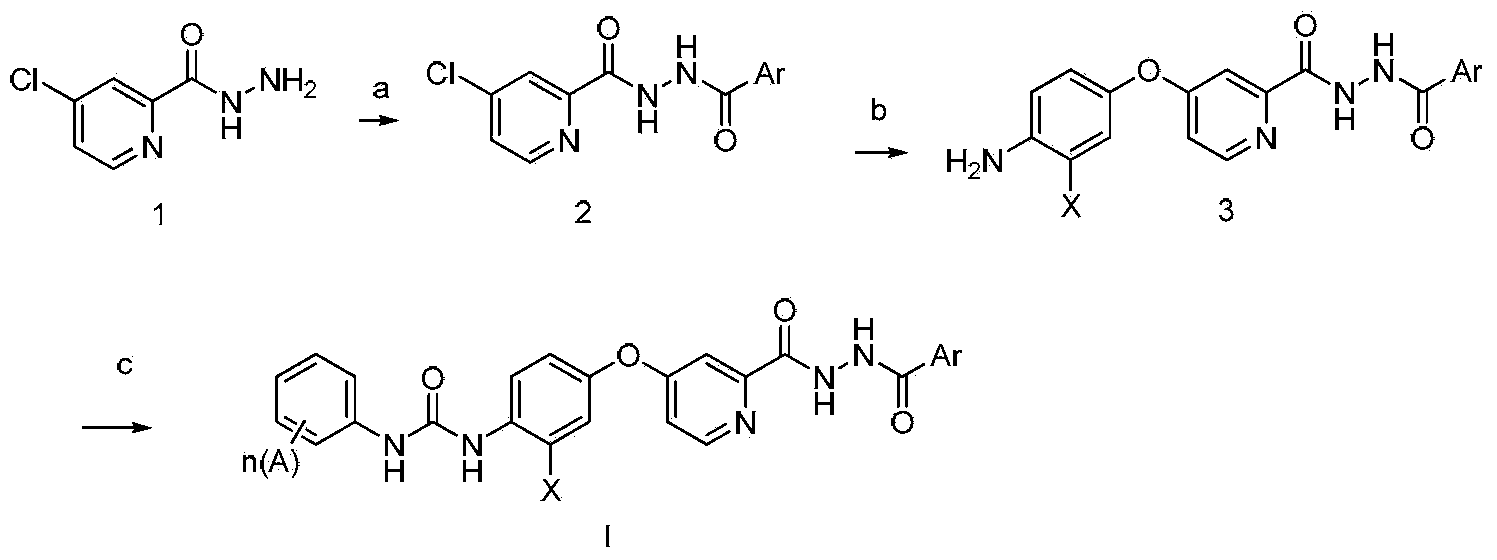
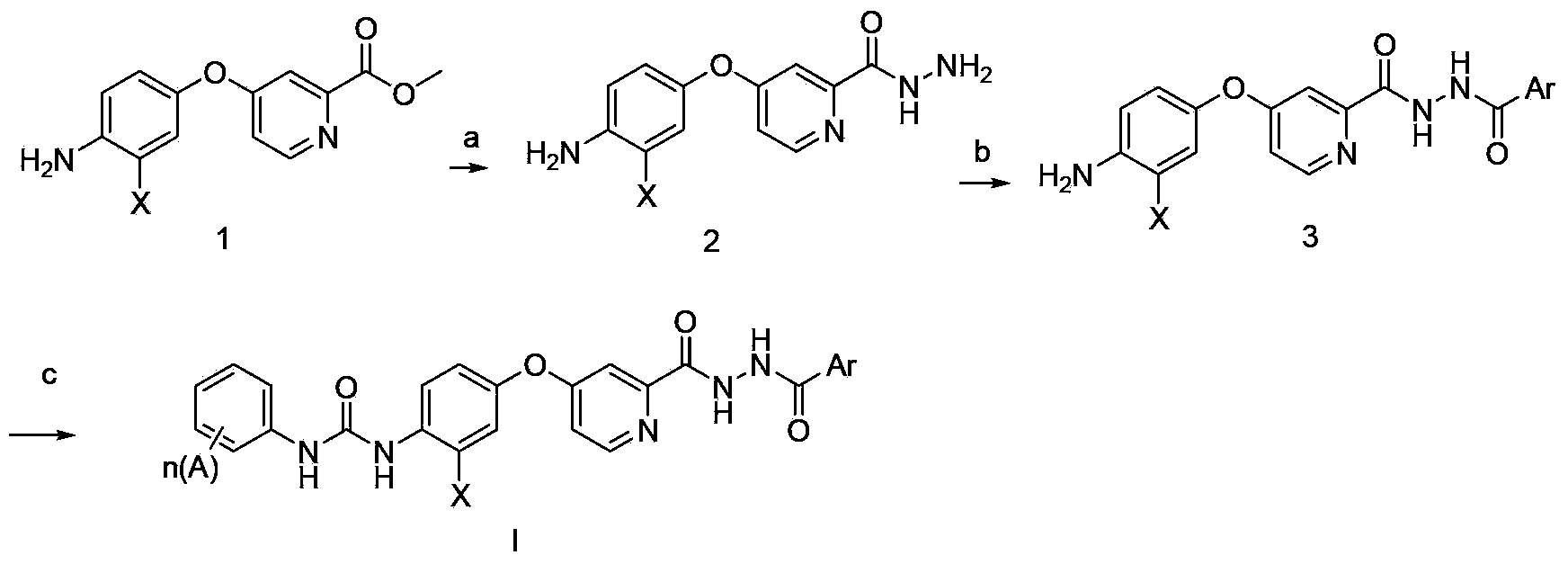
N'-aryl formyl o-pyridine hydrazide derivatives, and preparation methods, pharmaceutical composition and application thereof
A kind of arylformyl ortho, pyridine hydrazide technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1.1-(4-chloro-3-trifluoromethylphenyl)-3-(4-(2-(2-(p-methoxybenzoyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl ) urea
[0075]
[0076] Synthesis of N'-p-methoxybenzoyl-4-chloropyridine-2-hydrazide
[0077] Dissolve 1.1g (7.0mmol) of p-methoxybenzoic acid in 3mL DMF, add raw materials 1g (5.8mmol) of 4-chloropyridine-2-hydrazide, 2.7g (7.0mmol) of HATU, 1.8g of triethylamine ( 17.4mmol), stirred at room temperature, TLC monitored the complete reaction of raw materials, added 100mL of water, a large amount of off-white solid precipitated, and 1.67g of the product was obtained. 1 H NMR (400MHz, DMSO-d 6 ):10.68(s,1H,-CONH-),10.44(s,1H,-CONH-),8.70(d,1H,Ar-H),8.07(s,1H,Ar-H),7.90(d, 2H,Ar-H),7.84(d,1H,Ar-H),7.05(d,2H,Ar-H),3.84(s,3H,-O-CH 3 ).MS(FAB):(M + +1=306).
[0078] Synthesis of N'-p-methoxybenzoyl-4-(p-aminophenoxy)pyridine-2-hydrazide
[0079] Dissolve 469mg (4.3mmol) of p-aminophenol in 10mL of DMF, add 648mg (5.3mmol) of potassium tert-butoxide u...
Embodiment 2
[0081] Example 2.1-(4-(2-(2-(6-Hydroxynicotinoyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethylphenyl) Urea
[0082]
[0083] Using 6-hydroxynicotinic acid instead of p-methoxybenzoic acid, referring to the operation of Example 1, 175 mg of the target compound was obtained as a white solid. 1 H NMR (300MHz, DMSO-d 6 ):10.86(m,2H,-CONH-),9.33(s,1H,-CONH-),9.11(s,1H,-CONH-),8.53(dd,1H,Ar-H),8.12(m, 2H,Ar-H),7.86(d,1H,Ar-H),7.63(m,4H,Ar-H),7.36(m,1H,Ar-H),7.19(m,3H,Ar-H) ,6.38(d,1H,Ar-H).MS(FAB)(M + +1=587)
Embodiment 3
[0084] Example 3.1-(4-(2-(2-(pyridine-2-formyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethylphenyl ) urea
[0085]
[0086] Pyridine-2-carboxylic acid was used instead of p-methoxybenzoic acid, and the procedure of Example 1 was followed to obtain 135 mg of the target compound as a white solid. 1 H NMR (300MHz, DMSO-d 6 ):10.59(br,2H,-CONH-),9.39(s,1H,-CONH-),9.17(s,1H,-CONH-),8.69(s,1H,Ar-H),8.58(s, 1H,Ar-H),8.13(s,1H,Ar-H),8.04(s,2H,Ar-H),7.63(m,5H,Ar-H),7.41(s,1H,Ar-H) ,7.21(m,3H,Ar-H).MS(FAB)(M + +1=571)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com