Crystals of morphinan derivative, manufacturing method thereof, and pharmaceutical composition using the same
A manufacturing method, the technology of morphinan, applied to and using the pharmaceutical composition, the manufacturing method, the crystallization field, can solve the problems such as cumbersome, lengthy manufacturing method, low pH value change and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 6
[0042]First, 17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3-furyl)acrylamide] The morphinan was dispersed in a methanol solvent, and an anhydrous hydrochloric acid organic solution was added to form a second solution. Then, after stirring for 15 minutes, the second solution was concentrated and dried to obtain a 17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans - Crystallization of 3-(3-furyl)acrylamido]morphinan hydrochloride. The contents of each composition of Examples 1 to 6 are shown in Table 1.
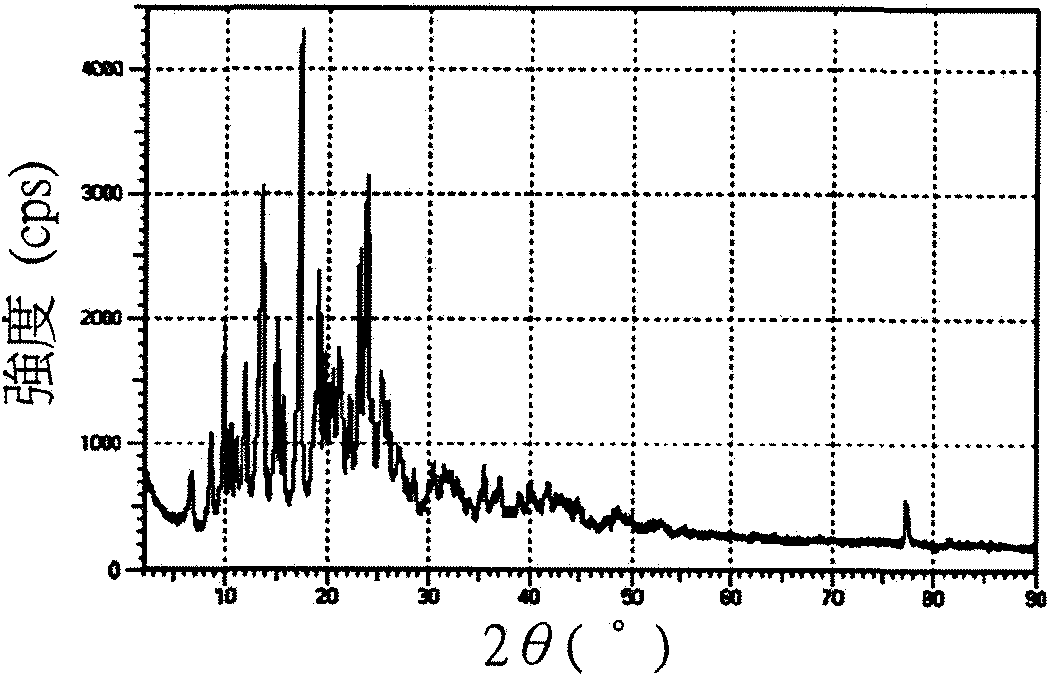
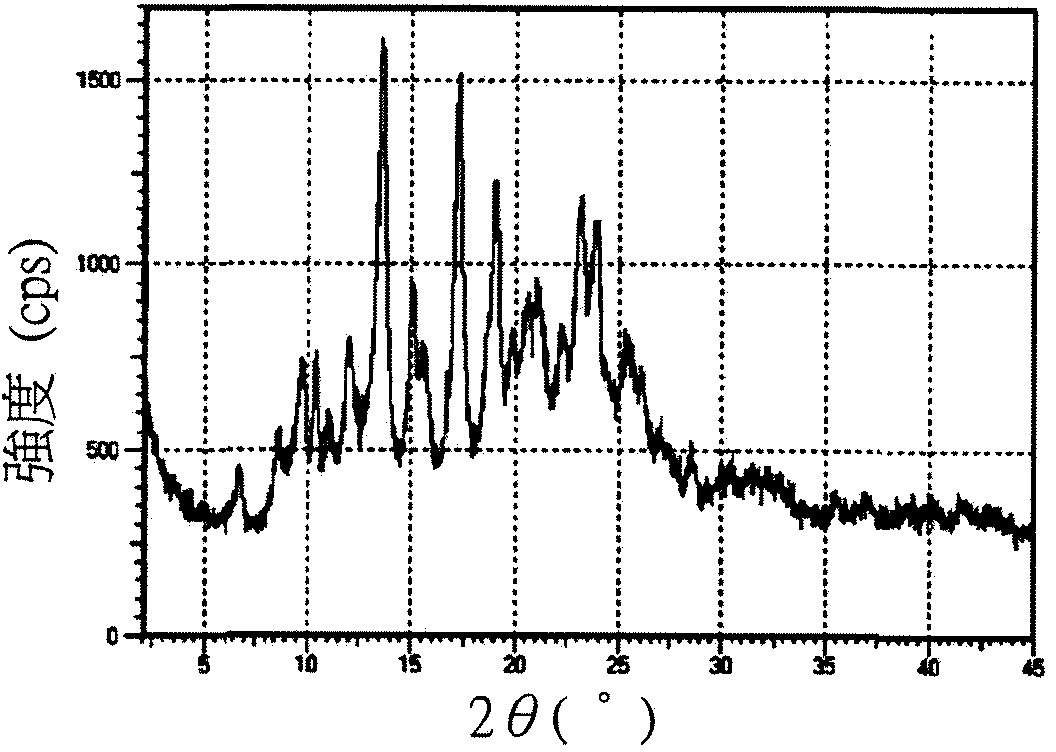
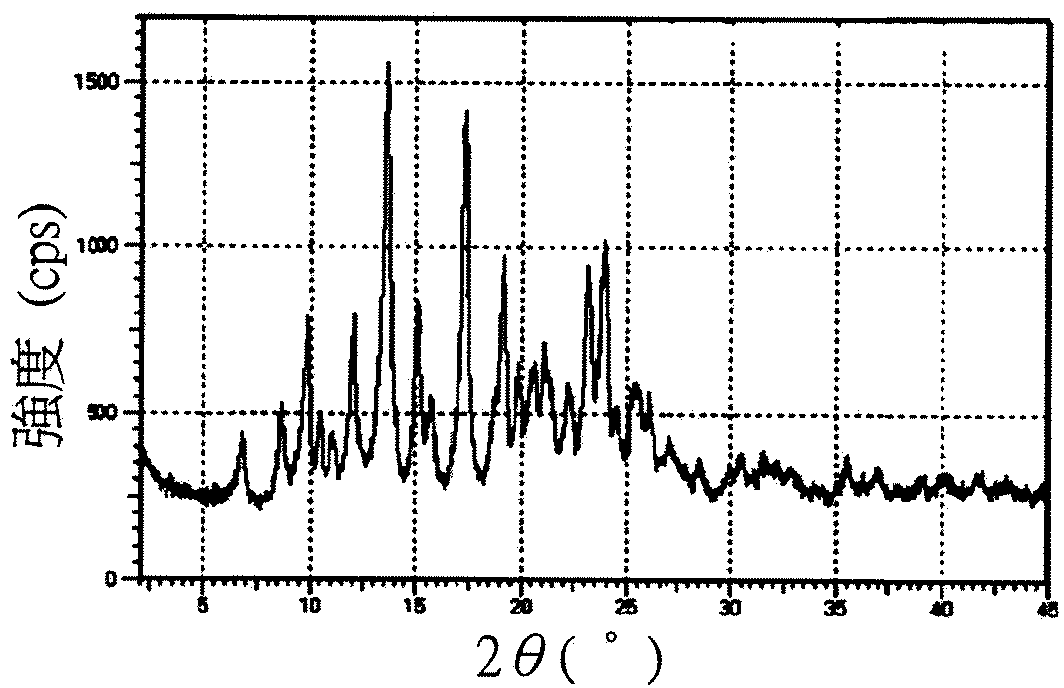
[0043] Please refer to Figures 1A to 1C , respectively 17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3 The X-ray powder diffraction pattern of the crystals of -furyl)acrylamido]morphinan salt. Please refer to Figure 2 to Figure 4 , respectively 17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3 X-ray powder diffraction pattern of -furyl)acrylamido]morphinan hydrochloride crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com