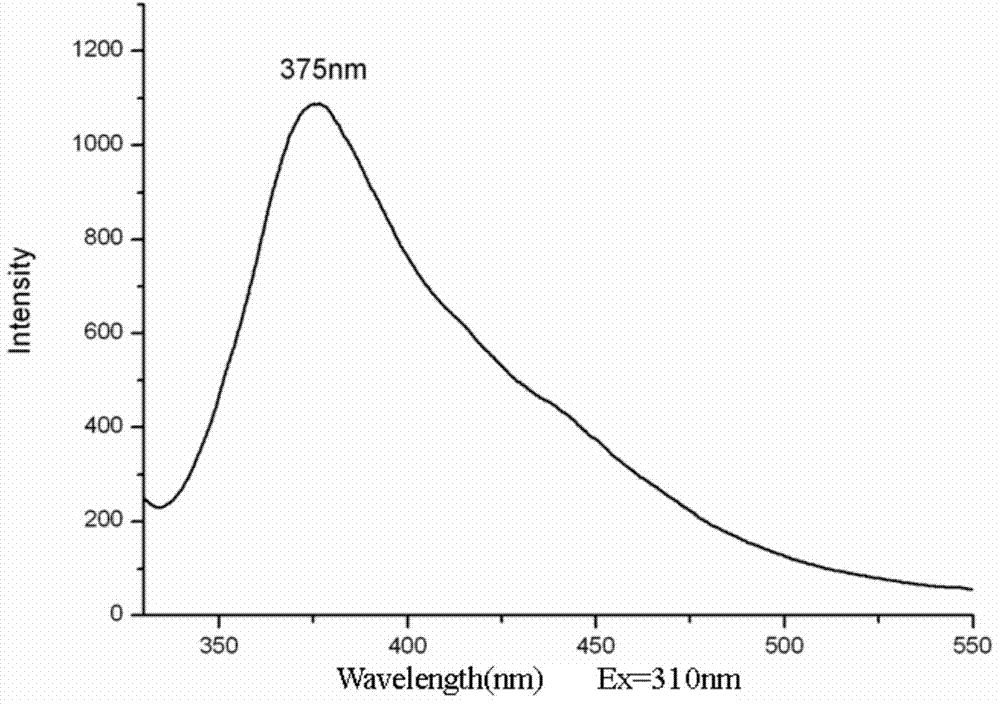
Purple fluorescent material and preparation method thereof
A technology of fluorescent materials and cadmium compounds, which is applied in the field of purple fluorescent materials and its preparation, can solve the problems such as the rare transition metal coordination and luminescence, and achieve a unique photoelectric activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
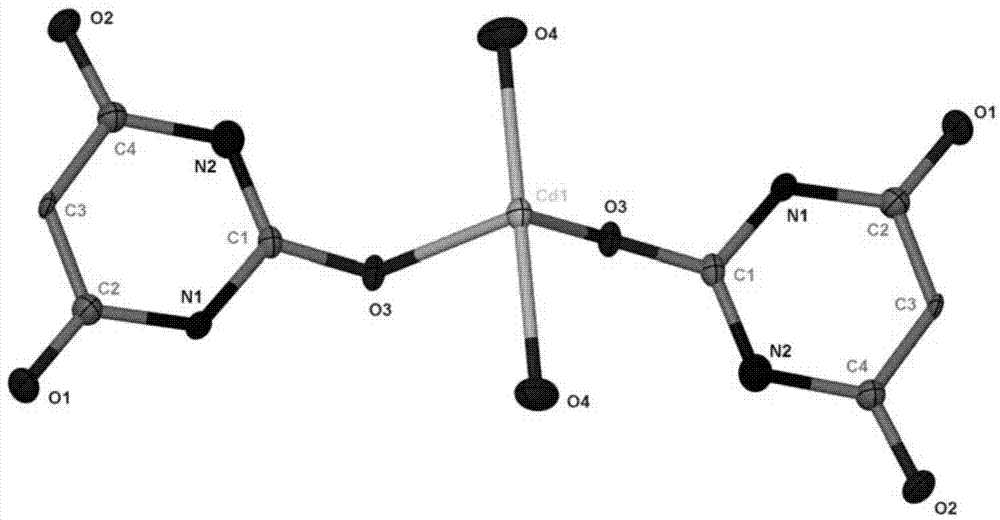
[0018] Weigh Cd(OAc) 2 2H 2 O (0.021g, 0.1mmol), barbituric acid (0.026g, 0.2mmol), heated and dissolved with 4ml of deionized water and cooled, placed in a 10ml test tube, added 3ml of ethanol dropwise to the test tube with a dropper, and used The test tube mouth is sealed by the parafilm, and several small holes are pierced on the parafilm. After the solution volatilizes for two weeks, a light yellow block crystal is separated out, which is the compound of the cadmium barbiturate.
Embodiment 2
[0020] Weigh Cd(OAc) 2 2H 2 O (0.021g, 0.1mmol), barbituric acid (0.013g, 0.1mmol), heated and dissolved with 2ml of deionized water and cooled, placed in a 10ml test tube, added 3ml of ethanol dropwise to the test tube with a dropper, and used The test tube mouth is sealed by the parafilm, and several small holes are pierced on the parafilm. After the solution volatilizes for two weeks, a light yellow block crystal is separated out, which is the compound of the cadmium barbiturate.
Embodiment 3
[0022] Weigh Cd(OAc) 2 2H 2 O (0.021g, 0.1mmol), barbituric acid (0.039g, 0.3mmol), heated and dissolved with 6ml of deionized water and cooled, placed in a 10ml test tube, added 3ml of ethanol dropwise to the test tube with a dropper, and used The test tube mouth is sealed by the parafilm, and several small holes are pierced on the parafilm. After the solution volatilizes for two weeks, a light yellow block crystal is separated out, which is the compound of the cadmium barbiturate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com