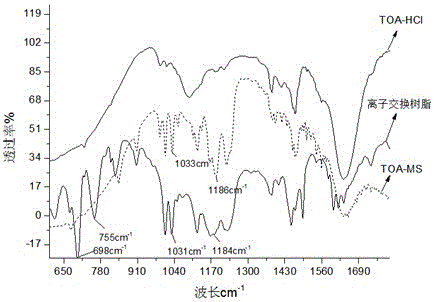
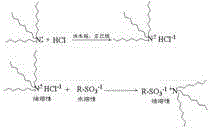
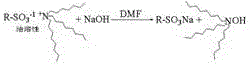A kind of strongly acidic cation exchange resin and preparation method thereof
A strong acid cation and exchange resin technology, which is applied in the field of functional polymer preparation, can solve the problems of waste acid discharge, pollution of the environment, waste of water resources, production safety, etc., and achieve high ion exchange efficiency, high density, and uniform distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A strong acid cation exchange resin, calculated in parts by weight, its raw material composition and content are as follows:
[0057] 34.7 parts of unsaturated alkyl sulfonate
[0058] Trioctylamine 55.2 parts
[0059] 24.5 parts of hydrochloric acid
[0060] 245.5 parts of n-hexane
[0061] Styrene 0 parts
[0062] Divinylbenzene 10.1 parts
[0063] Toluene 38.6 parts
[0064] Azobisisobutyronitrile 3.3 parts
[0065] Stabilizer 8.18 parts
[0066] N,N-Dimethylidene formamide 34.4 parts
[0067] 488.2 parts of sodium hydroxide
[0068] The unsaturated alkyl (aryl) sulfonate is sodium p-styrene sulfonate (MS88);
[0069] Described stabilizer gelatin and hydroxymethyl cellulose are the mixture that is formed by mass ratio of 10:1;
[0070] Described sodium hydroxide is the sodium hydroxide aqueous solution that concentration is 1mol / L;
[0071] Described hydrochloric acid is the hydrochloric acid aqueous solution that mass percent concentration is 36%;
[007...
Embodiment 2
[0087] A strong acid cation exchange resin, calculated in parts by weight, its composition and content are as follows:
[0088] Unsaturated alkyl (aryl) sulfonate 35.6 parts
[0089] Trioctylamine 56.5 parts
[0090] 25.2 parts of hydrochloric acid
[0091] 251.7 parts of n-hexane
[0092] Styrene 0 parts
[0093] Divinylbenzene 7.9 parts
[0094] Toluene 40.7 parts
[0095] Azobisisobutyronitrile 3.4 parts
[0096] Stabilizer 8.4 parts
[0097] N,N-Dimethylmethyleneformamide (DMF) 35.3 parts
[0098] 500.6 parts of sodium hydroxide
[0099] The unsaturated alkyl (aryl) sulfonate is sodium p-styrene sulfonate (MS88);
[0100] Described stabilizer gelatin and hydroxymethyl cellulose are the mixture that is formed by mass ratio of 10:1;
[0101] Described sodium hydroxide is the sodium hydroxide aqueous solution that concentration is 1mol / L;
[0102] Described hydrochloric acid is the hydrochloric acid aqueous solution that volume percent concentration is 36%;
[010...
Embodiment 3
[0116] A strong acid cation exchange resin, calculated in parts by weight, its composition and content are as follows:
[0117] Unsaturated alkyl (aryl) sulfonate 36.6 parts
[0118] Trioctylamine 58.3 parts
[0119] 25.5 parts of hydrochloric acid
[0120] 254.7 parts of n-hexane
[0121] Styrene 2.54 parts
[0122] Divinylbenzene 2.54 parts
[0123] Toluene 43.7 parts
[0124] Azobisisobutyronitrile 3.4 parts
[0125] Stabilizer 8.5 parts
[0126] N,N-Dimethylmethyleneformamide (DMF) 35.6 parts
[0127] 506.5 parts of sodium hydroxide
[0128] The unsaturated alkyl (aryl) sulfonate is sodium vinyl sulfonate;
[0129] Described stabilizer gelatin and hydroxymethyl cellulose are the mixture that is formed by mass ratio of 10:1;
[0130] Described sodium hydroxide is the sodium hydroxide aqueous solution that concentration is 1mol / L;
[0131] Described hydrochloric acid is the hydrochloric acid aqueous solution that volume percent concentration is 36%;
[0132] The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com