A kind of oligophenylene vinylene compound and its preparation method and application
A technology for oligophenylene vinylene compounds, which is applied in the field of oligopolyphenylene vinylene compounds and their preparation, can solve problems such as strong cytotoxicity, and achieve the effects of increasing water solubility, high yield, and satisfying preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
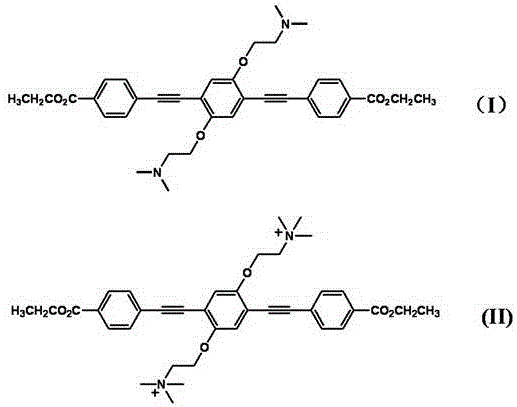
[0031] Example 1. Preparation of compound (I)
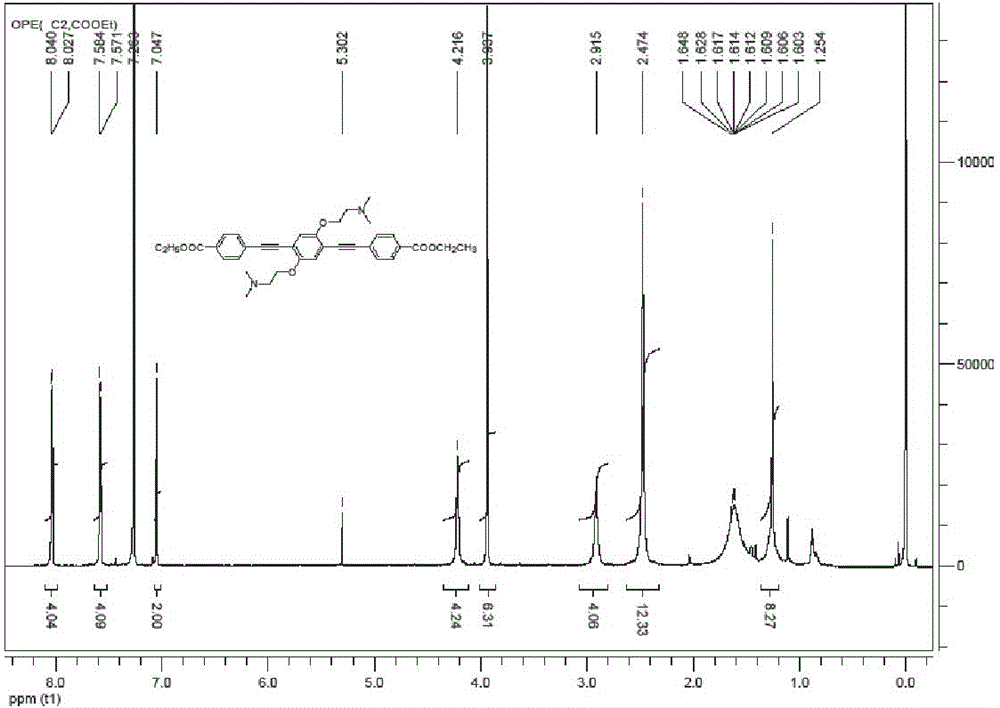
[0032]1 g (1.98 mmol) of OPE-1 was dissolved in 10 mL of anhydrous dichloromethane, and 396 mg (3.96 mmol) of diisopropylamine was added to obtain a colorless solution. 173 mg (0.99 mmol) e, 21 mg (0.03 mmol) (triphenylphosphine) palladium chloride, and 12 mg (0.06 mmol) cuprous iodide were sequentially added. After 7 hours, add 50 mL of dichloromethane to dilute the reaction system, add 20 mL of saturated ammonium chloride solution for washing, 20 mL of pure water and 20 mL of saturated sodium chloride, collect the organic phase, and wash with an appropriate amount of Dry magnesium sulfate with water for 30 minutes, remove magnesium sulfate by filtration, collect the organic phase, and remove the organic solvent by rotary evaporation under reduced pressure to obtain a light red solid. The solid was passed through a silica gel column to obtain a white solid (dichloromethane:methanol=10:1), yield: 75%. 1 H NMR (600 MHz, DMSO-d ...
Embodiment 2
[0033] Example 2. Preparation of compound (I)
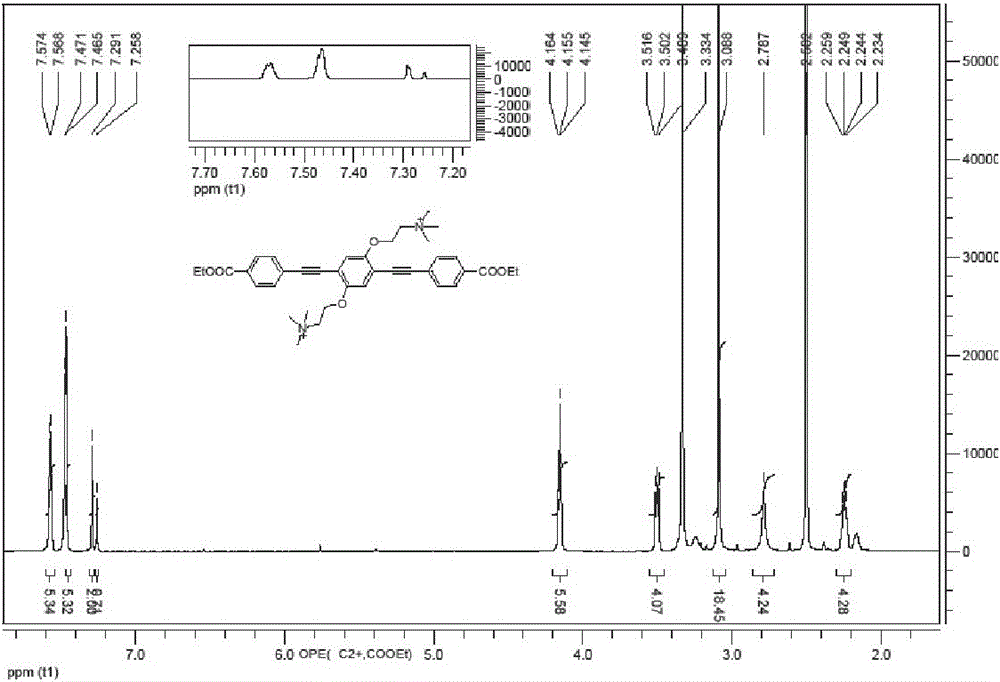
[0034] 1 g (1.98 mmol) of OPE-1 was dissolved in 10 mL of anhydrous dichloromethane, and 401 mg (3.96 mmol) of triethylamine was added to obtain a colorless solution. 173 mg (0.99 mmol) e, 21 mg (0.03 mmol) bis(triphenylphosphine) palladium chloride, and 12 mg (0.06 mmol) cuprous iodide were added sequentially. After 7 hours, add 50 mL of dichloromethane to dilute the reaction system, add 20 mL of saturated ammonium chloride solution to wash, 20 mL of pure water and 20 mL of saturated sodium chloride, collect the organic phase, and wash with an appropriate amount of anhydrous Magnesium sulfate was dried for 30 minutes, the magnesium sulfate was removed by filtration, the organic phase was collected, and the organic solvent was removed by rotary evaporation under reduced pressure to obtain a light red solid. The solid was passed through a silica gel column to obtain a white solid (dichloromethane:methanol=10:1), yield: 62%. 1 H ...
Embodiment 3
[0035] Example 3. Preparation of compound (I)
[0036] 1 g (1.98 mmol) of OPE-1 was dissolved in 10 mL of anhydrous dichloromethane, and 396 mg (3.96 mmol) of diisopropylamine was added to obtain a colorless solution. Add 173 mg (0.99 mmol) e, 42 mg (0.06 mmol) (triphenylphosphine) palladium chloride, and 24 mg (0.12 mmol) cuprous iodide in sequence. After 7 hours, add 50 mL of dichloromethane to dilute the reaction system, add 20 mL of saturated ammonium chloride solution for washing, 20 mL of pure water and 20 mL of saturated sodium chloride, collect the organic phase, and wash with an appropriate amount of Dry magnesium sulfate with water for 30 minutes, remove magnesium sulfate by filtration, collect the organic phase, and remove the organic solvent by rotary evaporation under reduced pressure to obtain a light red solid. The solid was passed through a silica gel column to obtain a white solid (dichloromethane:methanol=10:1), yield: 81%. 1 H NMR (600 MHz, DMSO-d 6 ). δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com