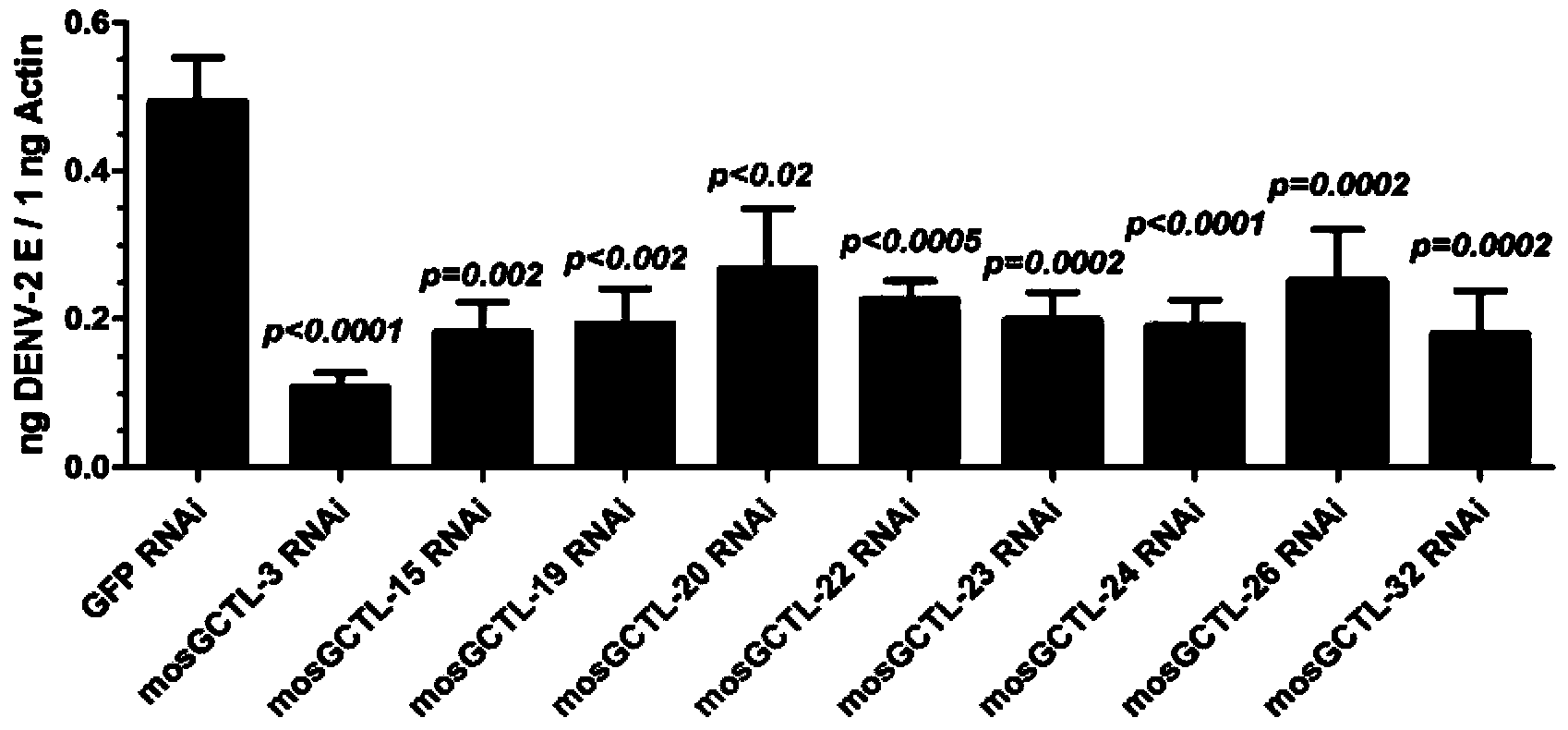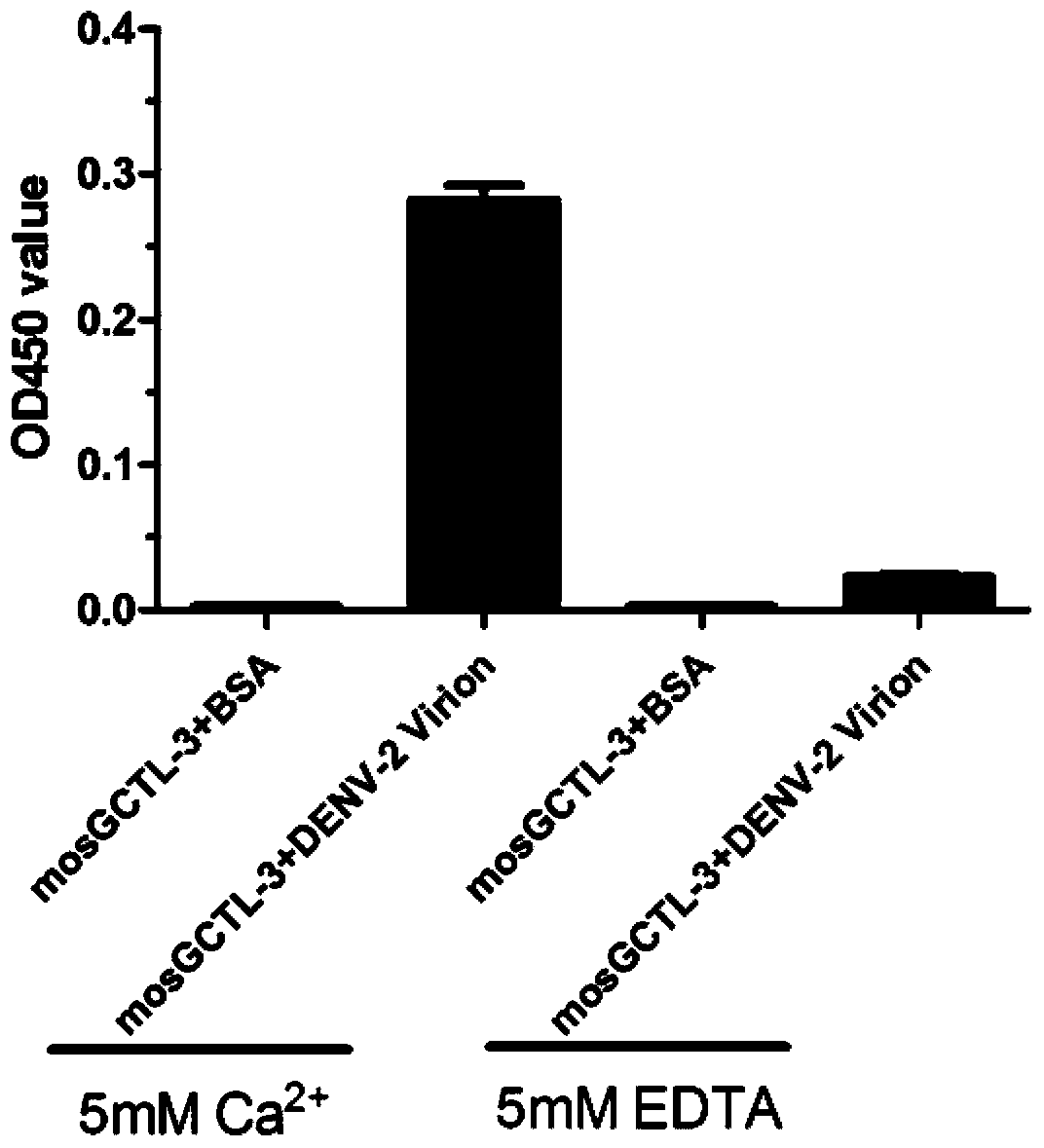Vaccine for preventing and/or treating dengue fever
A technology for dengue fever and dengue fever virus, applied in gene therapy, peptide/protein components, biochemical equipment and methods, etc., can solve the problems of reducing the safety of dengue fever vaccine, hindering the research and development of dengue fever vaccine, etc., and achieve the effect of reducing the virulence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Application of substances inhibiting mosGCTL-3 gene expression in inhibiting dengue virus replication
[0029] The C-type lectin isoform 3 of Aedes aegypti, also known as mosGCTL-3 protein, is shown in Sequence 1 of the Sequence Listing. The gene encoding the mosGCTL-3 protein is also called the mosGCTL-3 gene, and the coding region of its cDNA is shown in sequence 2 of the sequence listing.
[0030] 1. Design dsRNA
[0031] The dsRNA (dsRNA-1) designed to inhibit the expression of the mosGCTL-3 gene, that is, the double-stranded RNA corresponding to the double-stranded DNA shown in nucleotides 1 to 333 from the 5' end of Sequence 2 of the Sequence Listing.
[0032] A dsRNA (dsRNA-2) designed to inhibit GFP gene expression, the double-stranded RNA shown in SEQ ID NO: 19 of the Sequence Listing.
[0033] 2. Application of substances that inhibit the expression of mosGCTL-3 gene in inhibiting the replication of dengue fever type 2 virus
[0034] 1. Divide Ae...
Embodiment 2
[0046] Example 2. Application of substances inhibiting mosGCTL-26 gene expression in inhibiting dengue virus replication
[0047] The C-type lectin isoform 26 of Aedes aegypti is also known as mosGCTL-26 protein, as shown in sequence 3 of the sequence listing. The gene encoding the mosGCTL-26 protein is also called the mosGCTL-26 gene, and the coding region of its cDNA is shown in sequence 4 of the sequence listing.
[0048] 1. Design dsRNA
[0049] The dsRNA (dsRNA-3) designed to inhibit the expression of the mosGCTL-26 gene, that is, the double-stranded RNA corresponding to the double-stranded DNA shown in nucleotides 31-381 from the 5' end of sequence 4 of the sequence listing.
[0050] 2. Application of substances inhibiting the expression of mosGCTL-26 gene in inhibiting the replication of dengue fever type 2 virus
[0051] 1. Divide Aedes aegypti into two groups and deal with them as follows:
[0052] Experimental group: each Aedes aegypti mosquito was injected with 2...
Embodiment 3
[0063] Example 3. Application of substances inhibiting mosGCTL-15 gene expression in inhibiting dengue virus replication
[0064] The C-type lectin subtype 15 of Aedes aegypti is also called mosGCTL-15 protein, as shown in SEQ ID NO: 5 of the sequence listing. The gene encoding the mosGCTL-15 protein is also called the mosGCTL-15 gene, and the coding region of its cDNA is shown in Sequence 6 of the Sequence Listing.
[0065] 1. Design dsRNA
[0066] The dsRNA (dsRNA-4) designed to inhibit the expression of the mosGCTL-15 gene, that is, the double-stranded RNA corresponding to the double-stranded DNA shown in nucleotides 79 to 407 from the 5' end of sequence 6 of the sequence listing.
[0067] 2. Application of substances that inhibit the expression of mosGCTL-15 gene in inhibiting the replication of dengue fever type 2 virus
[0068] 1. Divide Aedes aegypti into two groups and deal with them as follows:
[0069] Experimental group: each Aedes aegypti mosquito was injected wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com