A kind of preparation method and application of dengue fever double-effect vaccine
A technology for dengue fever and vaccine products, which is applied in the field of preparation of dengue virus double-effect vaccines, can solve the problems of non-specific therapeutic therapy and safe and effective dengue fever vaccine, and achieve the effect of reducing bleeding and death, and reducing the rate of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1, the acquisition of DENV2 ΔNS1 protein
[0053] Express DENV2 NS1 and DENV2 △NS1 proteins through prokaryotic, as follows:
[0054] 1. Acquisition of DENV2 NS1 gene and DENV2 △NS1 gene
[0055] 1) DENV2 NS1 gene
[0056] Extract the total RNA of DENV2 virus, reverse transcribe to obtain cDNA as a template, and use F1 and R1 primers to PCR amplify to obtain a 1056bp PCR product, which is the DENV2 NS1 gene. After sequencing, its nucleotide sequence is sequence 1, and its The encoded protein is DENV2 NS1, the amino acid length of the protein is 352AA, and the amino acid sequence is sequence 2.
[0057] 2) DENV2 △NS1 gene
[0058] The total RNA of DENV2 virus was extracted, and the cDNA obtained by reverse transcription was used as a template for amplification.
[0059] The first step: using the cDNA of the DENV2 virus as a template, carry out PCR amplification with the primer pair of F2 and R2, and obtain the PCR product of 1-115AA; Amplify to obtain a PCR ...
Embodiment 2
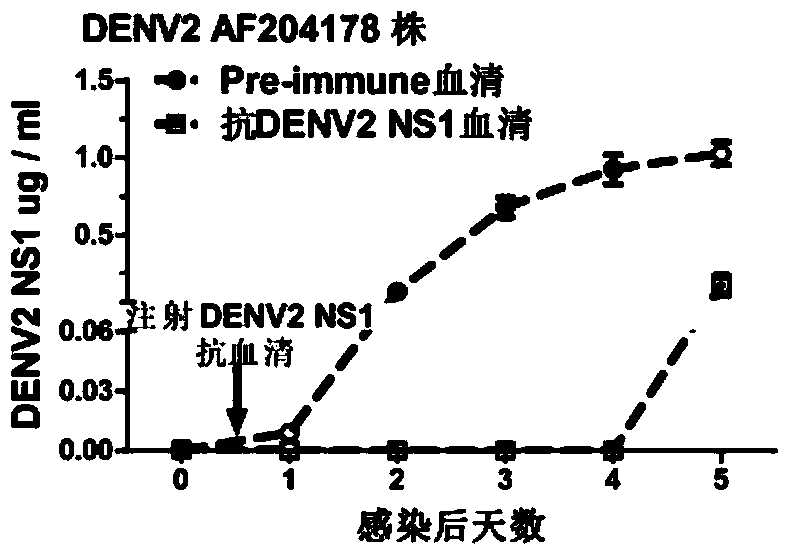
[0090] Example 2, the application of DENV2 NS1 and DENV2△NS1 in passive immunization to hinder the acquisition of dengue virus
[0091] 1. Preparation of DENV2 NS1 mouse-derived antiserum and DENV2 ΔNS1 mouse-derived antiserum
[0092] 1. Preparation of mouse antiserum.
[0093] The experimental animals were 8-week-old Balb / c female mice, and the experimental procedure was as follows:
[0094] (1). On the first day (initial immunization), each mouse was injected intraperitoneally with an equal-volume mixture of the DENV2 NS1 and DENV2 △NS1 proteins prepared in the above-mentioned one and Freund's complete adjuvant respectively (containing 40ug of DENV2 NS1 or DENV2 △NS1 protein ).
[0095] (2). On the 14th day (the first booster immunization), each mouse was subcutaneously injected with an equal-volume mixture of the DENV2 NS1 and DENV2 ΔNS1 proteins prepared above and Freund's incomplete adjuvant (containing 40ug DENV2 NS1 or DENV2 ΔNS1 NS1 protein).
[0096] (3). On the ...
Embodiment 3
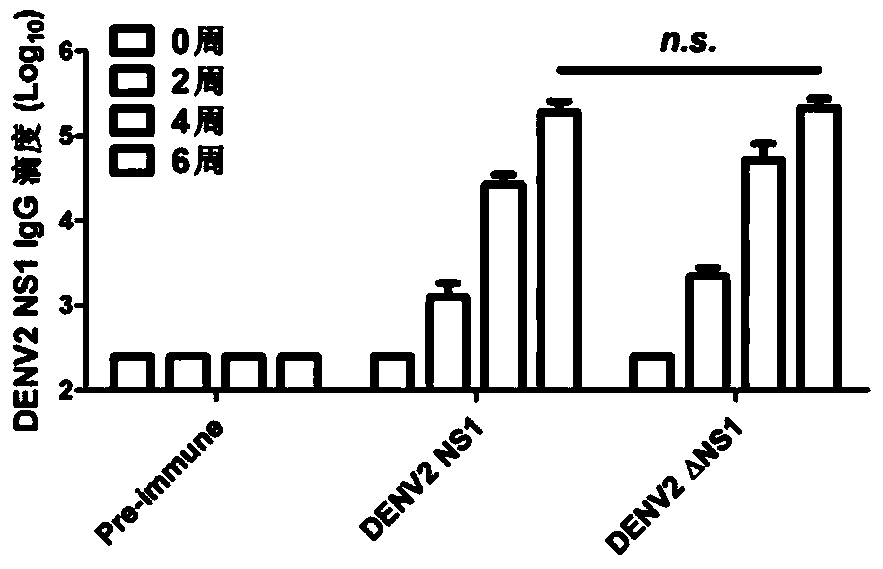
[0123] Embodiment 3, the application of DENV2 NS1 and DENV2 △ NS1 as dengue fever vaccine
[0124] 1. Active immunization of AG6 mice with DENV2 NS1 protein and DENV2 △NS1 protein
[0125] The DENV2 NS1 protein and DENV2 △NS1 protein prepared in Example 1 were actively immunized in AG6 mice. The immunized mice were 6-week-old AG6 mice. The experiment was divided into three groups, PBS control group, DENV2 NS1 and DENV2 △NS1 Experimental group, 12 AG6 mice in each group, the immunization method is as follows:
[0126] 1. Day 1 (primary immunization)
[0127] DENV2 NS1 experimental group: each AG6 mouse was intraperitoneally injected with an equal volume mixture of DENV2 NS1 prepared in Example 1 and Freund's complete adjuvant (40ugDENV2 NS1 per mouse);
[0128] DENV2 ΔNS1 experimental group: each AG6 mouse was intraperitoneally injected with an equal volume mixture of DENV2 ΔNS1 prepared in Example 1 and Freund's complete adjuvant (40ug DENV2 ΔNS1 per mouse);
[0129] Contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com