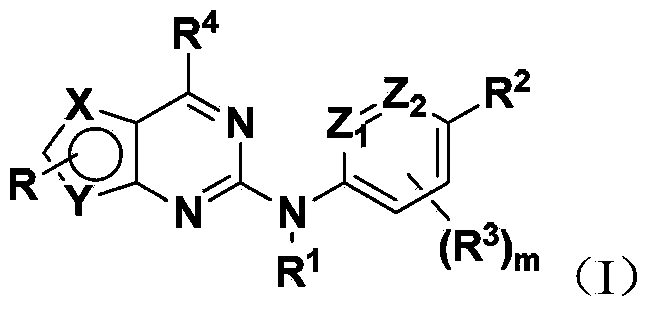
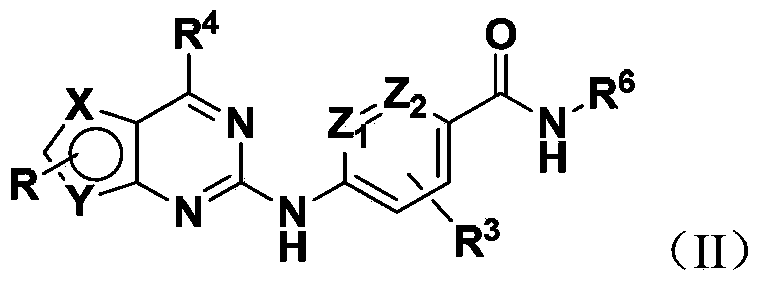
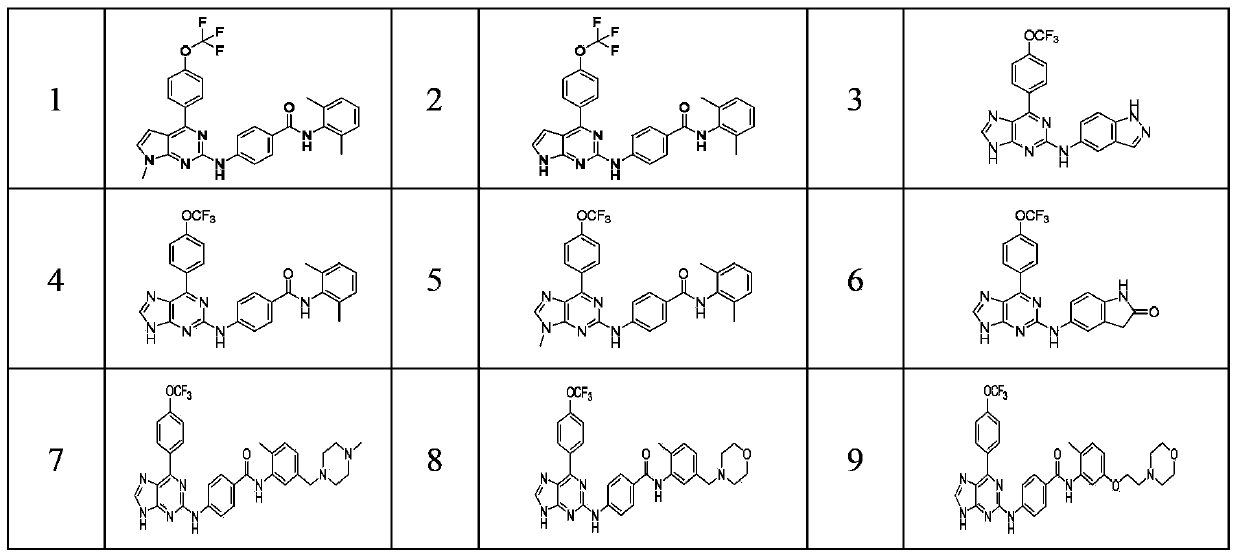
Bicyclic heterocyclic amine Hedgehog signal pathway inhibitor
A heterocycloalkyl and heteroaryl technology, applied in the field of heterocyclic amine Hedgehog signaling pathway inhibitors, can solve the problems of narrow indications and incomplete development of the medical value of Hh inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
[0135] Preparation of compound 1 and compound 2
[0136]
[0137] Preparation of 2-chloro-4-(4-(trifluoromethoxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidine (intermediate 1-a)
[0138] Weigh 2,4-dichloro-7H-pyrrolopyrimidine (372mg, 1eq), p-trifluoromethoxyphenylboronic acid (1000mg, 1.1eq), triethylamine (1.2eq), bis(triphenylphosphine) Palladium(II) chloride (0.1eq), N,N-dimethylformamide (28.5ml) and water (0.5ml) were added into the flask and heated to 85°C for 4h. After stopping the reaction, add 100ml of water to the reaction solution, stir, extract with ethyl acetate (20ml*4), dry the ester layer with anhydrous sodium sulfate, concentrate, and separate by column chromatography (petroleum ether:ethyl acetate=20:1 ) to give a solid (680mg, 41%). 1 H-NMR (400M, DMSO-d 6 )δ12.56(s,1H,NH),8.31(d,2H,ArH),7.76(d,1H,ArH),7.60(d,2H,ArH),6.98(d,1H,ArH)ppm.
[0139] Preparation of 2-chloro-7-p-toluenesulfonyl-4-(4-(trifluoromethoxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidine (intermediate ...
Embodiment 3
[0148] Preparation of compound 3
[0149]
[0150] Preparation of 2,6-dichloro-9-(4-methoxybenzyl)-9H-purine (intermediate 3-a)
[0151] Dissolve 2,6-dichloro-9H-purine (1g, 5.29mmol) and 1-chloro-4-methoxybenzene (0.91g, 5.82mmol) in N,N-dimethylformamide (10ml) , add potassium carbonate (0.88g, 6.35mmol), react at room temperature for 17 hours, add ethyl acetate to dilute, wash with water, dry, filter, concentrate the filtrate to make sand, and separate by column chromatography (petroleum ether / ethyl acetate=2:1) Intermediate 3-a (823 mg, 50%) was obtained. MS(ESI)m / z:[M+H] + =309.2. 1 H-NMR (400M, DMSO-d 6 )δ8.82(s,1H,ArH),7.33(d,2H,ArH),6.92(d,2H,ArH),5.41(s,2H,CH 2 ),3.73(s,3H,CH 3 ) ppm.
[0152] Preparation of 2-chloro-9-(4-methoxybenzyl)-6-(4-(trifluoromethoxy)phenyl)-9H-purine (intermediate 3-b)
[0153] Weigh intermediate 3-a (760mg, 2.46mmol), 4-trifluoromethoxyphenylboronic acid (760mg, 3.69mmol), bis(triphenylphosphine)palladium(II) chloride dichloromet...
Embodiment 4
[0159] Preparation of Compound 4
[0160]
[0161] Methyl 4-(9-(4-methoxybenzyl)-6-(4-(trifluoromethoxy)phenyl)-9H-purin-2-ylamino)benzoate (4-a) preparation of
[0162] Weigh intermediate 3-b (546mg, 1.26mmol), methyl p-aminobenzoate (285mg, 1.88mmol), palladium acetate (28mg, 0.12mmol), 2,2'-bisdiphenylphosphino-1,1 '-Binaphthyl (156mg, 0.24mmol), cesium carbonate (1.23g, 3.78mmol), add dioxane (15ml), microwave at 150°C for 5 hours, add water, extract with ethyl acetate, dry, filter, The filtrate was concentrated to make sand, and separated by column chromatography (petroleum ether / ethyl acetate=10:1) to obtain intermediate 4-a (200 mg, 29%). MS(ESI)m / z:[M+H] + =550.1. 1 H-NMR (400M, DMSO-d 6 )δ10.18(s,1H,NH),8.93(d,2H,ArH),8.56(s,1H,ArH),8.01(d,2H,ArH),7.94(d,2H,ArH),7.63( d,2H,ArH),7.40(d,2H,ArH),6.94(d,2H,ArH),5.41(s,2H,CH 2 ),3.84(s,3H,CH 3 ),3.72(s,3H,CH 3 ) ppm.
[0163] Preparation of 4-(9-(4-methoxybenzyl)-6-(4-(trifluoromethoxy)phenyl)-9H-purin-2-ylami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com