Methods related to treatment of inflammatory diseases and disorders
A disease, autoimmune disease technology with application in the related field of treatment of inflammatory diseases and disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0254] 1. A method for predicting a subject's response to an anti-inflammatory drug comprising: obtaining in a biological sample from said subject figure 1 Information about the expression level of one or more genes, wherein the change in the expression level of one or more genes compared to the reference level of the gene predicts the subject's response to the anti-inflammatory drug .
[0255] 2. Methods for predicting patient response to anti-inflammatory drugs, including:
[0256] a. Detection in a biological sample from said patient figure 1 the expression level of one or more genes, and
[0257] b. Comparing said level to a reference level for said gene
[0258] wherein the altered expression of one or more of said genes compared to said reference level predicts said patient's response to said anti-inflammatory drug.
[0259] 3. A method for identifying a subject with an increased likelihood of responding to an anti-inflammatory drug, comprising: obtaining in a biolog...
Embodiment 1
[0439] Blood samples from Phase 1b and Phase 2a trials examining the safety, tolerability and efficacy of anti-IL-20 in patients with rheumatoid arthritis (RA) were collected from patients at time points (Clinical Trials Government Identification numbers: NCT01038674 and NCT01282255): pre-dose (Day 1) and post-dose days 8, 15, 29, 43, and 99 in the Phase 1b trial, and pre-dose (Day 1 ) and the 15th and 36th day after administration. The patients were dosed weekly for 6 weeks (total of 7 doses) and weekly for 11 weeks (total of 12 doses), respectively.
[0440] Total RNA was obtained as described above. After reduction of globin mRNA and confirmation of RNA integrity, RNA samples were analyzed by AffyMetrix GeneChip hybridization following the procedure described above.
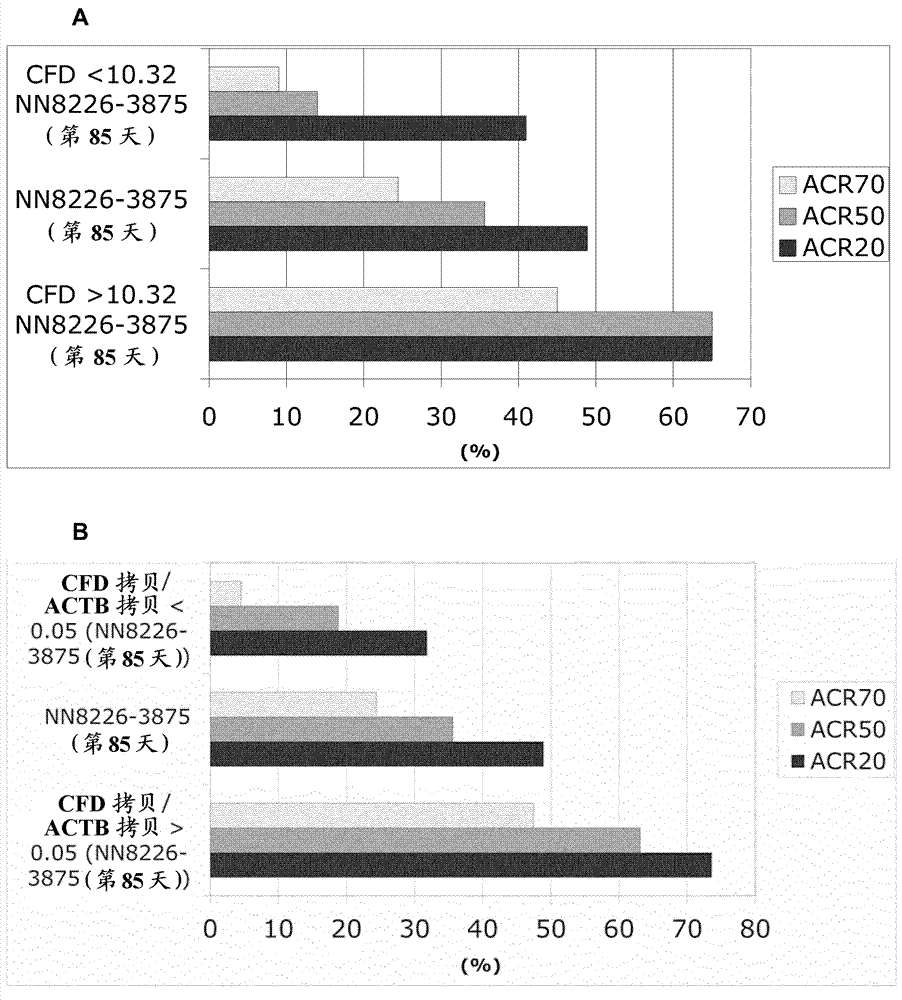
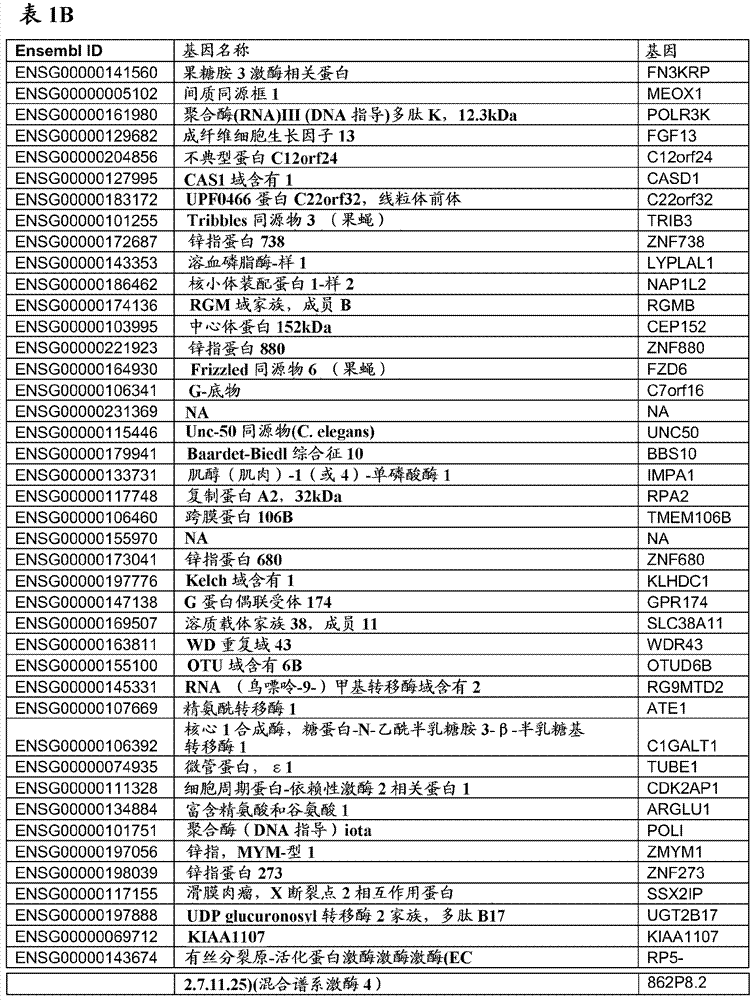
[0441] To identify transcripts in whole blood PaxGene samples that correlate with changes in DAS28-CRP and other disease score measures such as ACR20, ACR50, and ACR70, we performed regression analysis on ex...
Embodiment 2
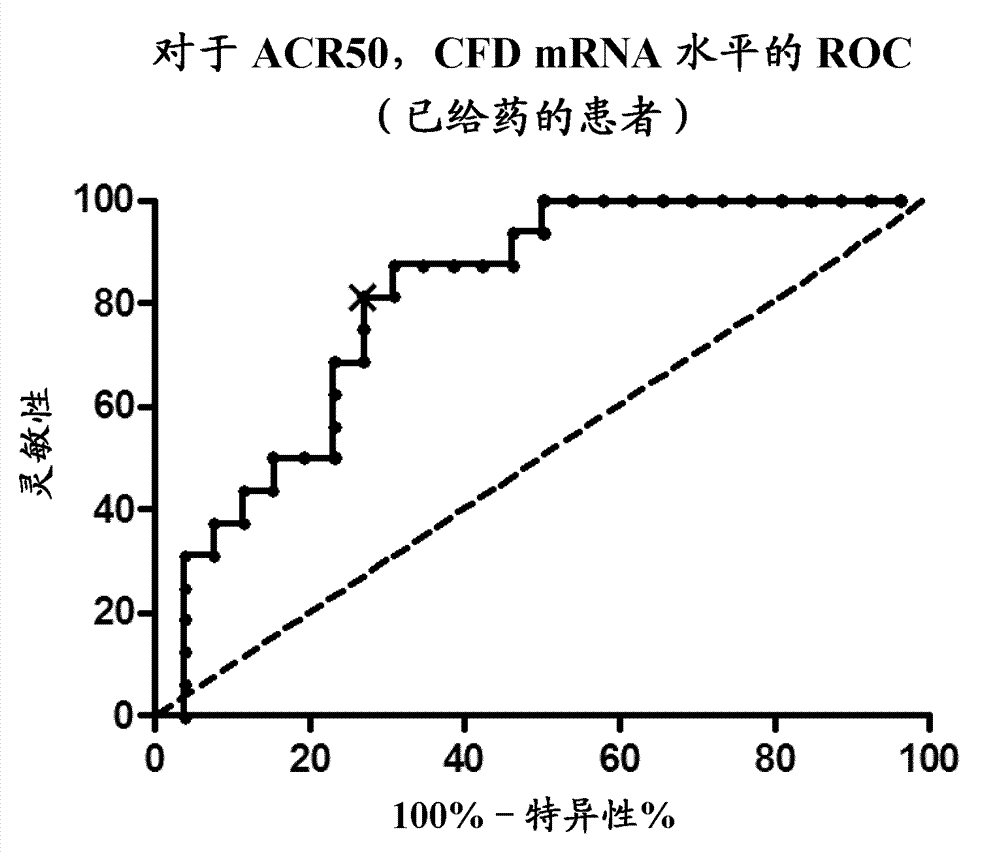
[0450] To test whether baseline (pre-dose) determination of CFD mRNA alone correlates with CFD mRNA levels from different time points assessed in the microarray analysis, qRT-PCR was performed on CFD mRNA on available pre-dose samples, and the These correlate with recorded CFD levels from the microarray. Quantitative RT-PCR analysis was performed as described above and data were obtained from duplicate analyzes of each cDNA sample. CFD mRNA levels from the qRT-PCR platform were normalized to 18S rRNA levels also detected by qRT-PCR. To correlate microarray levels with qRT-PCR levels, RNA-normalized microarray levels were transformed to a linear scale. like Figure 6 As shown, a high association was found between qRT-PCR assays at baseline and microarray assays from several visits (R 2 =0,86). This suggests that CFD-based stratification of RA-patients at baseline (before dosing) is feasible because figure 1 and figure 2 Other predictive transcripts described in e.g. CFD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com