Forming method of nickel-metal hydride battery
A chemical formation method and technology of nickel-metal hydride batteries, which are applied in secondary batteries, alkaline batteries, electrochemical generators, etc., can solve the problems of high low-voltage ratio, inability to ship, and high battery self-discharge rate, and achieve high voltage. The effect of retention time, improved OCV retention, and improved charge retention ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
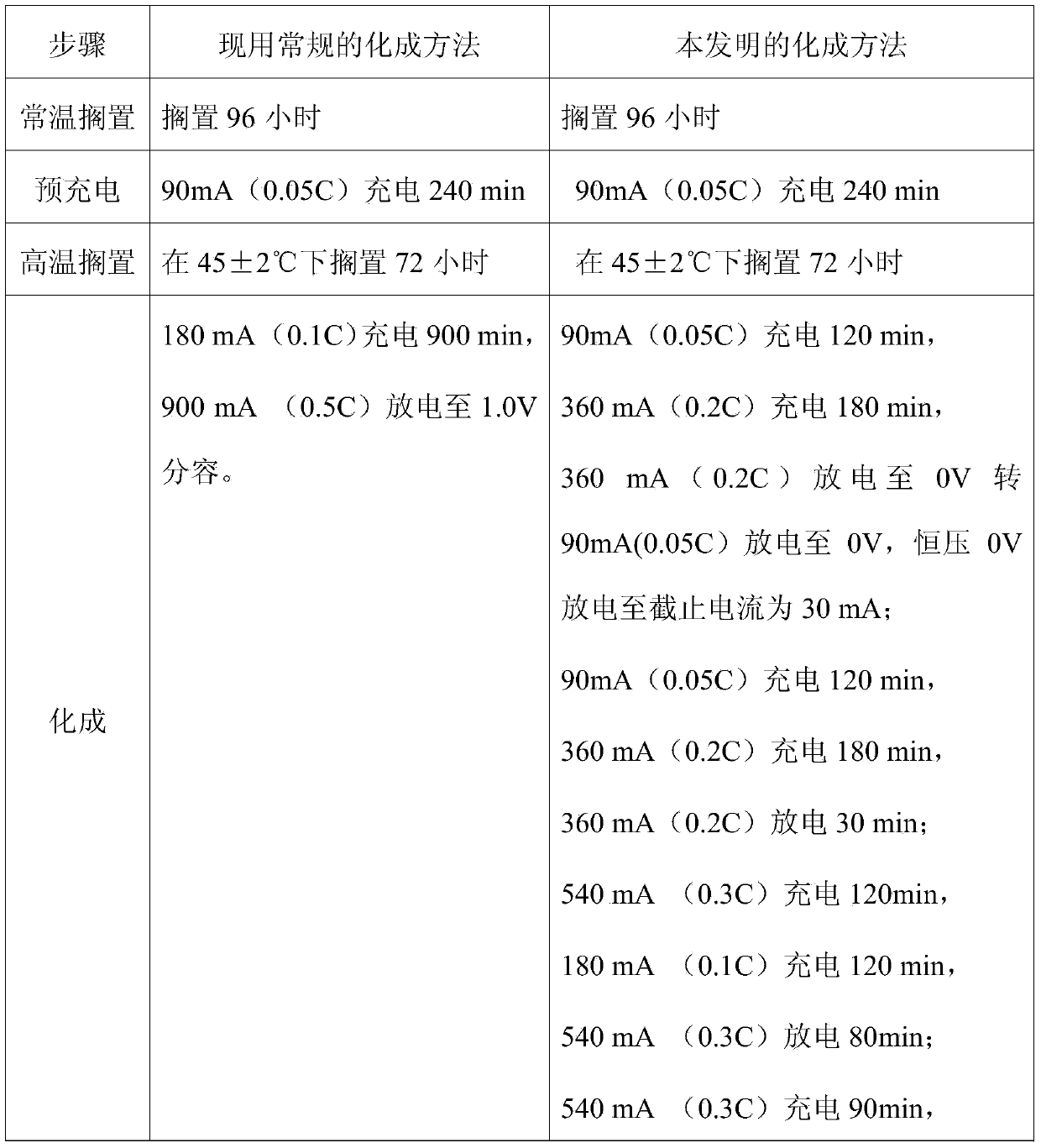
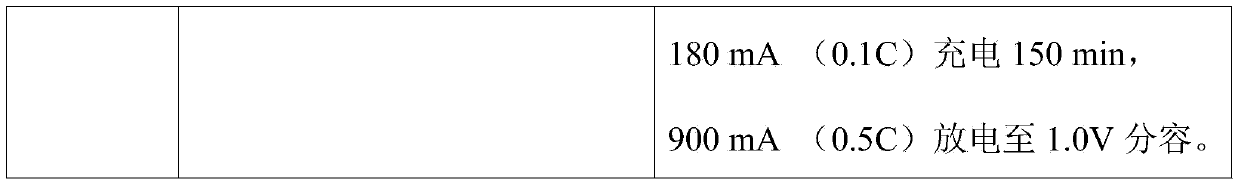
[0012] The assembled nickel-metal hydride AA1800 battery is formed on the Jiaxin secondary battery test cabinet through the current conventional formation method and the formation method of the present invention. The specific process steps are shown in Table 1.
[0013] Table 1 Two different formation methods
[0014]
[0015]
[0016] Randomly select 30 batteries produced by the two formation methods in Table 1 and conduct a 28-day charge retention test according to the standard of IEC61951-2. The results are shown in Table 2. As can be seen from Table 2, the charge retention rate of the battery produced by the chemical formation method of the present invention is 4.6% higher than that of the battery produced by the existing conventional chemical process.
[0017] Table 2 Comparison of 28-day charge retention rate
[0018]
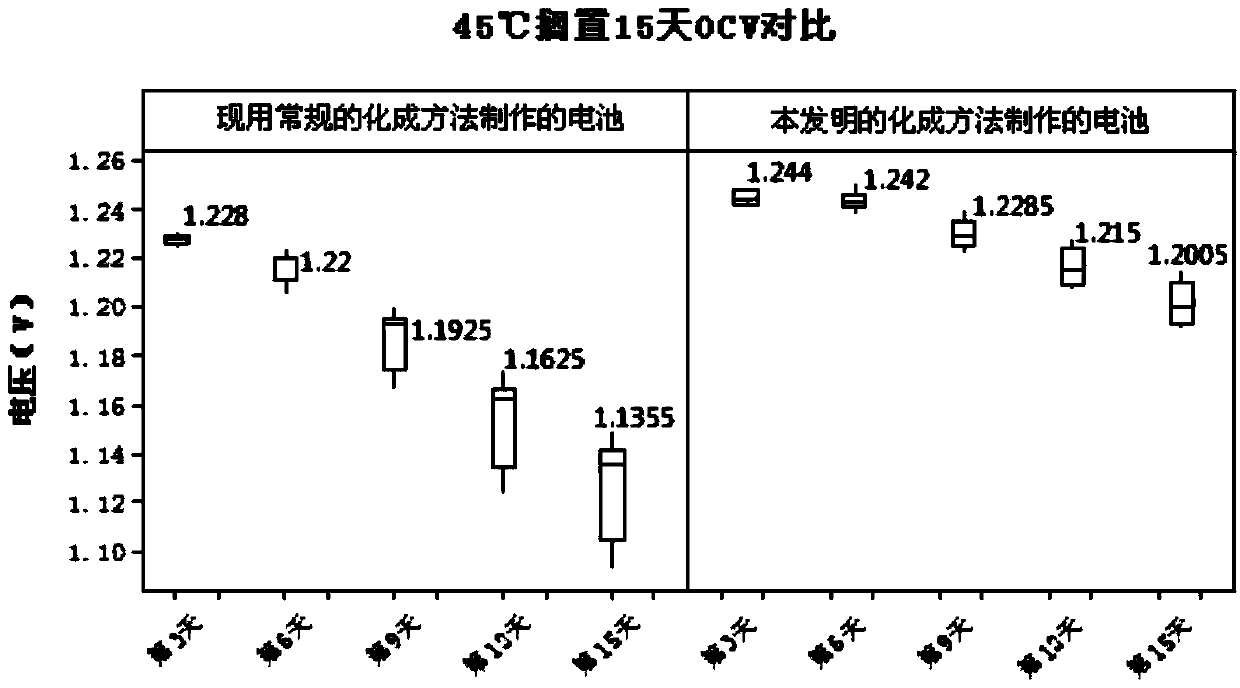
[0019] Randomly select 300 batteries made by the two formation methods in Table 1, and put them aside for 15 days in an environment of 45±2°C w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com