Preparing method for manganese nitrate solution
A technology of nitric acid aqueous solution and manganese nitrate, applied in the direction of manganese nitrate, etc., can solve the problems of slow reaction rate and increased cost, and achieve the effect of increasing reaction rate and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of manganese nitrate solution provided by the present invention, its basis reaction equation is:
[0018] 3Mn+8HNO 3 →3Mn(NO 3 ) 2 +2NO+4H 2 o
[0019] In the embodiment of the present invention, in order to facilitate observation and judge the end time of the preparation reaction, it is necessary to ensure the complete reaction of nitric acid and manganese metal; therefore, the molar ratio of manganese metal to nitric acid in the nitric acid aqueous solution is 3:8. In this way, when the metal manganese is completely dissolved, it can be determined that the reaction is complete.
Embodiment 1
[0021] Add 3.0042g of 30% hydrogen peroxide aqueous solution into 8.1011g of 58% nitric acid aqueous solution and stir evenly to obtain a two-component oxidation solution, wherein the nitric acid has a mass fraction of 42.31%, and the peroxidized The mass fraction of hydrogen is 8.12%;
[0022] Weigh 1.5400 g of manganese metal, add it into the two-component oxidation solution, and react to obtain a pink manganese nitrate solution. The reaction time is 150 minutes, and the mass fraction of the manganese nitrate solution is 53.27%.
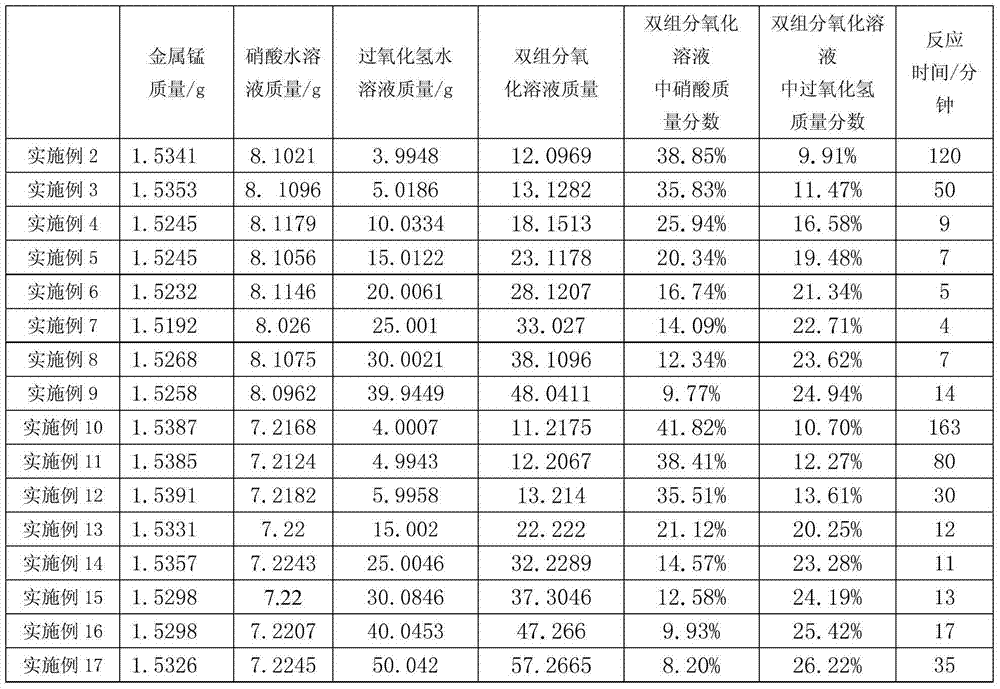
Embodiment 2~17
[0024] The manganese nitrate solutions of Examples 2 to 17 were prepared respectively by using the reactants and their amounts in Table 1, with reference to the preparation method described in Example 1, and the reaction times were recorded in Table 1.
[0025] The reactant kind, reactant consumption and reaction time of table 1 embodiment 2~17
[0026]
[0027] Note: the massfraction of nitric acid in the nitric acid aqueous solution used among the embodiment 2~9 is 58%; The massfraction of nitric acid in the nitric acid aqueous solution used among the embodiment 10~17 is 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com