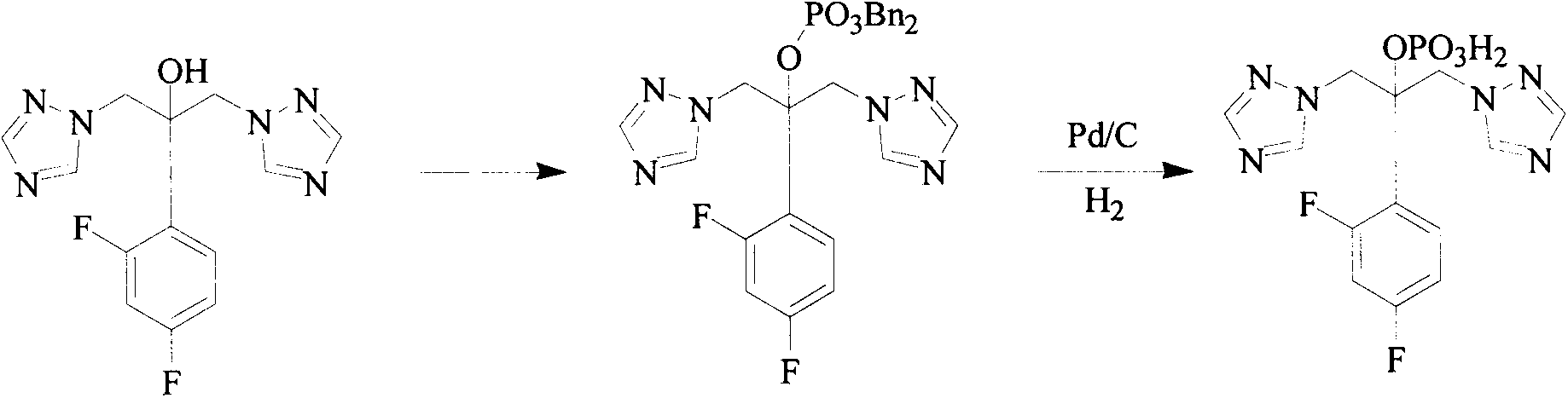
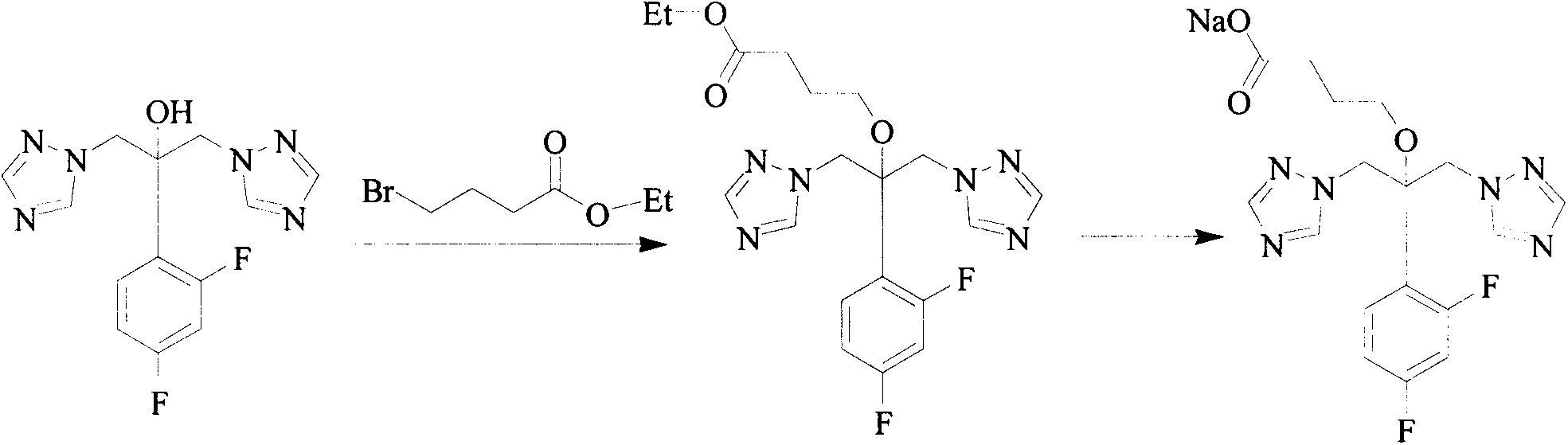
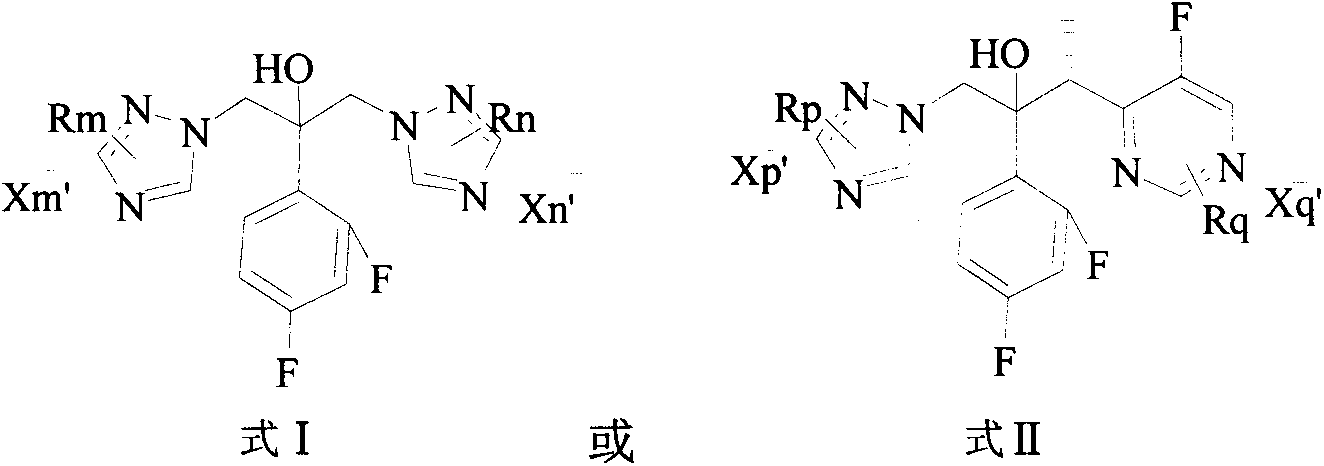
Triazole compound
A compound and triazole technology, applied in the field of triazole compound and the preparation of the compound, can solve the problems of side reaction, complex composition of compound and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of compound Ia
[0033]
[0034] In a 1000mL reaction flask, fully dissolve chloromethyl-2-methoxyethyl carbonate (42g, 0.25mol) and voriconazole (69.9g, 0.2mol) with 600mL acetonitrile solution, heat to reflux and react for 10h. After TLC detection, the reaction was concentrated to dryness, dichloromethane was added to dissolve the concentrate fully under heating conditions, cooled to room temperature, filtered to dryness and vacuum dried for a period of time to obtain a white solid, namely compound Ia (82g, 85%).
[0035] MS(ESI): 483.2([M+H] + ).
Embodiment 2
[0036] Embodiment 2: the synthesis of compound Ib
[0037]
[0038] According to the operation of Example 1, replace chloromethyl-2-methoxyethyl carbonate with chloromethyl-2-[2-methoxyethoxy] ethyl carbonate, and obtain white solid (Ib , 80%).
[0039] MS (ESI): 526.5 ([M+H] + ).
Embodiment 3
[0040] Embodiment 3: the synthesis of compound Ic
[0041]
[0042] According to the operation of Example 1, replace chloromethyl-2-methoxyethyl carbonate with chloromethyl-2-[2-[2-methoxyethoxy]ethoxy]ethyl carbonate after the reaction is complete after the post-treatment A white solid (Ic, 82%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com