Efficient serum-free culture medium
A serum-free medium and high-efficiency technology, applied in the direction of vertebrate cells, artificial cell constructs, animal cells, etc., can solve the problems of high cost and reduced expression, and achieve the effect of increasing growth, increasing expression, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, preparation of serum-free medium
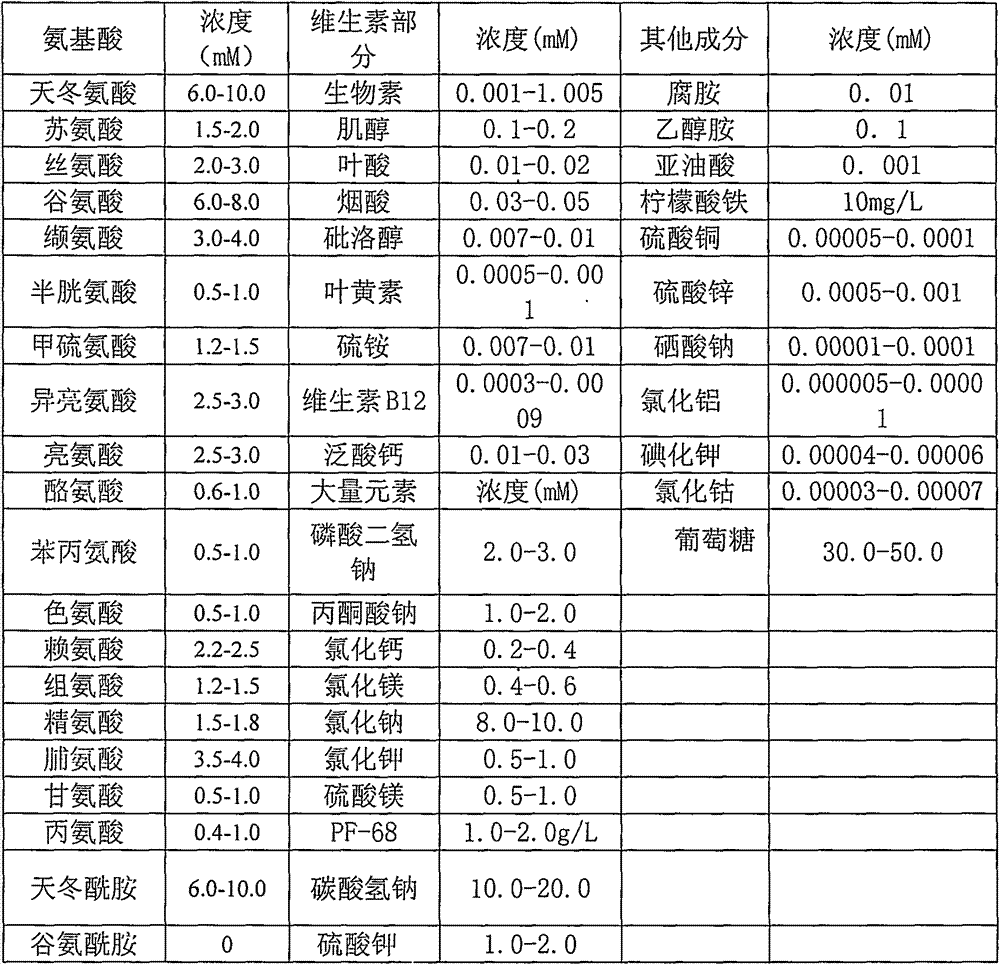
[0034] Table 1. Serum-free medium formulations
[0035]
[0036] Dissolve each component in pyrogen-free ultrapure water according to the formula in Table 1, and filter and sterilize to prepare the serum-free medium of the present invention.
Embodiment 2
[0037] Embodiment 2, optimizing glutamic acid, asparagine, the impact of aspartic acid concentration on cell growth
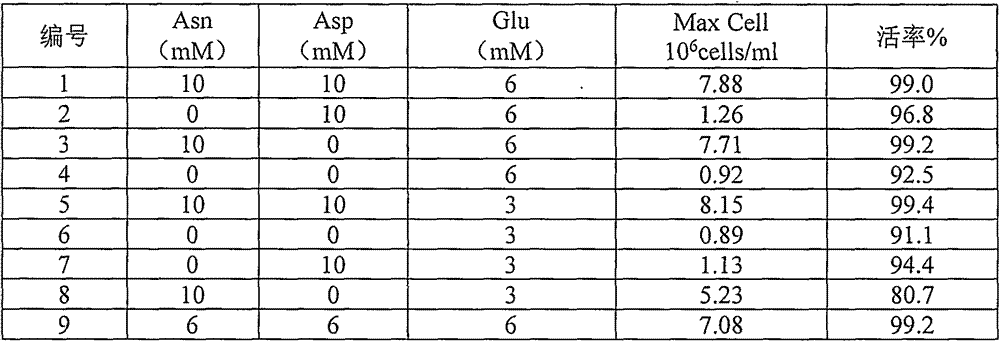
[0038] While maintaining the other nutrient components of the culture medium in accordance with Table 1, prepare culture media corresponding to different concentrations of glutamic acid, asparagine, and aspartic acid (Table 2). CHO cell lines expressing trastuzumab were treated according to 5x10 5 The concentration of cells / ml was inoculated, and cultured at 37 degrees, 110 rpm, and carbon dioxide concentration of 6.0%. When the glucose concentration was lower than 2 g / l, the sugar was added to 8 g / l. Cultured for 14 days, the number of cells reached the maximum on the 8th day, and Asp10mM / Asn10mM / Glu3mM produced the maximum cell density of 8.16x106 cells / ml. Asp6mM / Asn6mM / Glu6mM produced a cell density of 7.08x106 cells / ml (Table 2).
[0039]Table 2
[0040]
Embodiment 3
[0041] Example 3 Optimizing Glutamic Acid, Asparagine, and the Effect of Aspartic Acid Concentration on Cell Expression of Antibody
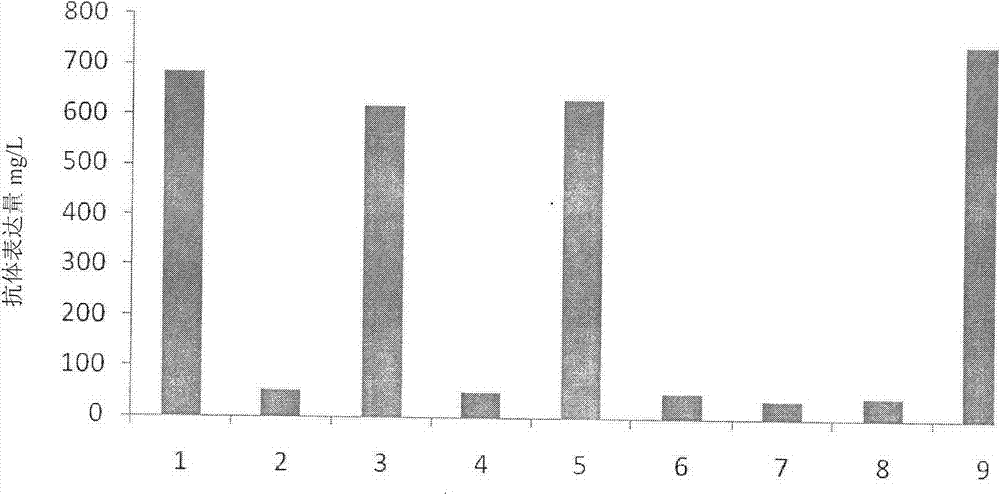
[0042] While maintaining the other nutrient components of the culture medium in accordance with Table 1, prepare culture media corresponding to different concentrations of glutamic acid, asparagine, and aspartic acid (Table 2). The CHO cell line expressing trastuzumab was inoculated at a concentration of 5x105 cells / ml, and cultured at 37 degrees, 110 rpm, and a carbon dioxide concentration of 6.0%. When the glucose concentration was lower than 2 g / L, supplement Sugar to 8 g / l. After culturing for 14 days, samples were taken on D14 to measure the expression level, wherein Asp6mM / Asn6mM / Glu6mM produced the highest expression level of 720 mg / L ( figure 1 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com