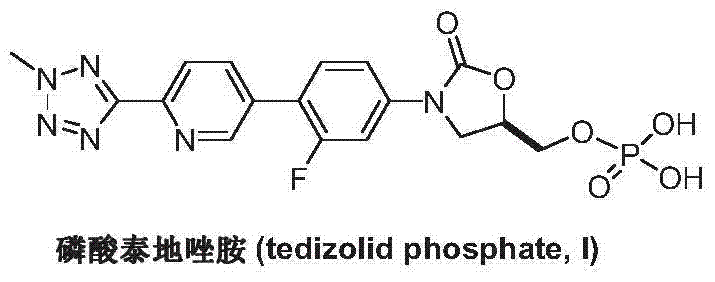
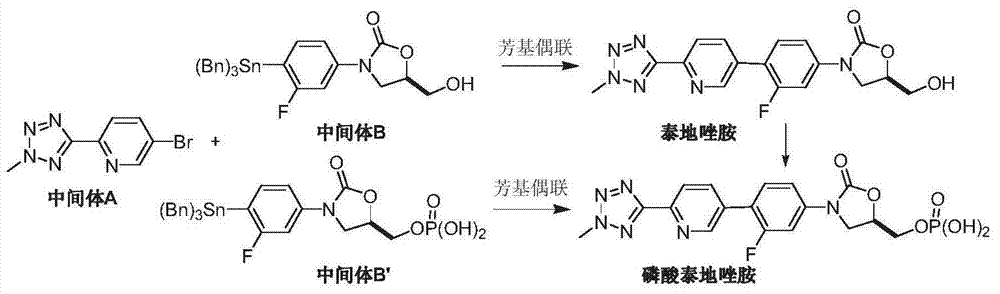
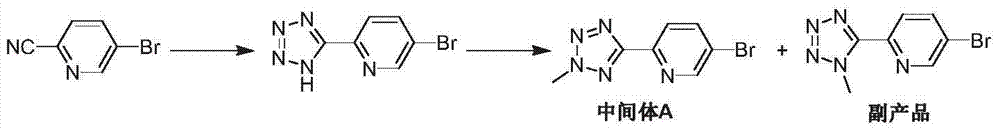
Preparation method of tedizolid phosphate
A technology of tedizolid phosphate and preparation steps, which is applied in the field of preparation of oxazolidinone antibiotic tedizolid phosphate, can solve the problems of difficulty in obtaining raw materials, increased side reactions, long preparation steps, etc., and achieves environmental protection of the process The effect of economy, promotion of development, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Under nitrogen atmosphere, add 2-(2-methyltetrazol-5-yl)pyridine-5-boronic acid (II) (2.15g, 10.5mmol), R-3-(3-fluoro- 4-iodo-phenyl)-2-oxo-5-oxazolidinylmethanol phosphate (III) (4.17g, 10mmol), tetrakis(triphenylphosphine)palladium (0.23g, 0.2mmol), 1M phosphoric acid Potassium solution 15mL and toluene 30mL were heated up to reflux, kept at reflux for 10-12 hours, and TLC detected that the reaction was complete. Add 30 mL of ethyl acetate, wash with water and saturated brine successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The resulting oil is recrystallized with n-hexane and ethyl acetate (1:1, V / V), and dried in vacuo to obtain a white Solid tedizolid phosphate (I) 3.82g, yield 84.9%, 1 H NMR (DMSO-d6): d 8.92(s, 1H), 8.20(m, 2H), 7.74(t, 1H), 7.66(dd, 1H), 7.50(dd, 1H), 4.95(m, 1H) , 4.46(s, 3H), 4.21(t, 1H), 4.05(m, 2H), 3.91(m, 1H), FAB-MS m / z: 451[M+H] + .
Embodiment 2
[0031]
[0032] Under a nitrogen atmosphere, 2-(2-methyltetrazol-5-yl)pyridine-5-boronic acid pinacid (II) (3.01g, 10.5mmol), R-3-(3 -Fluoro-4-bromo-phenyl)-2-oxo-5-oxazolidinylmethanol phosphate (III) (3.69g, 10mmol), [1,1'-bis(diphenylphosphino)dicene Iron] Palladium dichloride / dichloromethane complex (0.15g, 0.2mmol), potassium acetate (1.17g, 12mmol) and 50mL of 1,4-dioxane, heated to 110°C, stirred for 4-5 hours , TLC detected that the reaction was complete. Add 50 mL of ethyl acetate, wash with water and saturated brine successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The resulting oil is recrystallized with n-hexane and ethyl acetate (1:1, V / V), and dried in vacuo to obtain a white Solid tedizolid phosphate (I) 4.02g, yield 89.3%.
Embodiment 3
[0034]
[0035] Under a nitrogen atmosphere, 2-(2-methyltetrazol-5-yl)-5-bromo-pyridine (IV) (2.4g, 10mmol), bispinacol diborane ( 1.27g, 5mmol), 1,1'-bis(diphenylphosphino)ferrocenepalladium dichloride (0.82g, 1mmol), potassium acetate (1.17g, 12mmol), and 1,4-dioxane 30mL , the temperature was raised to 110° C., and the reaction was stirred for 4 hours. Cool down to room temperature, still under nitrogen atmosphere, add R-3-(3-fluoro-4-bromo-phenyl)-2-oxo-5-oxazolidinylmethanol phosphate (III) (3.69 g, 10 mmol), 20 mL of 1,4-dioxane and 0.5 mL of 5M potassium phosphate, the temperature was raised to 100°C again, and the reaction was stirred for 4 hours, and the reaction was detected by TLC. Add 50 mL of ethyl acetate, filter, the filtrate is washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated under reduced pressure, the obtained oil is recrystallized with n-hexane and ethyl acetate (1:1, V / V), vacuum After drying, 3.34 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com