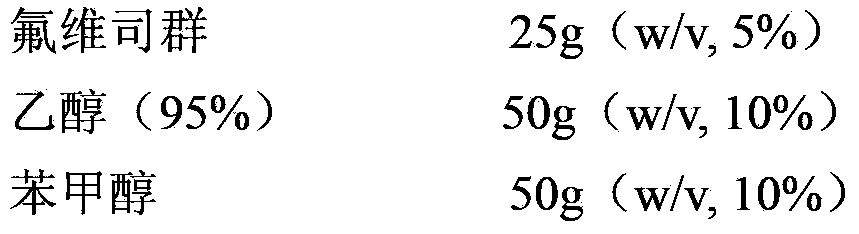Fulvestrant pharmaceutical composition
A kind of technology of fulvestrant and composition, applied in the field of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
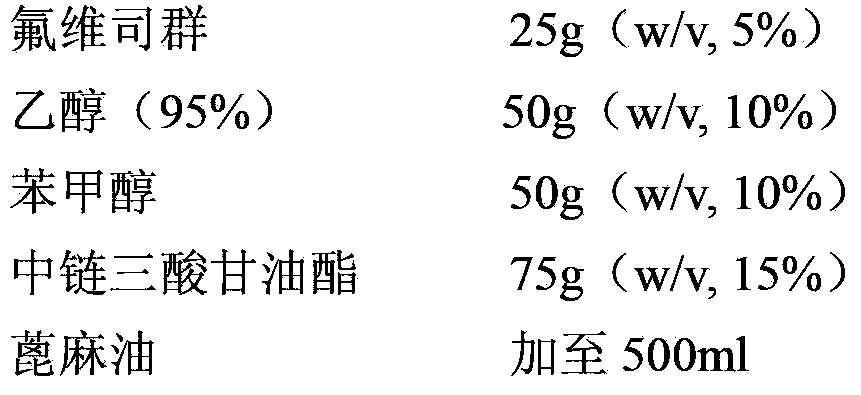
[0024] Embodiment 1: Preparation of 50mg / ml fulvestrant injection
[0025]
[0026] Preparation: Weigh the prescribed amount of fulvestrant, ethanol (95%) and benzyl alcohol and mix, stir until fulvestrant is completely dissolved; add the prescribed amount of medium-chain triglycerides to the above solution, stir, and mix well ; Add the prescribed amount of castor oil to the final volume (500ml), stir and mix. Filter the mixed solution through a 0.2 μm filter for 1 to 2 times to sterilize. In sterile, N-filled 2 Under protection, dispense samples into vials or prefilled syringes.
Embodiment 2
[0027] Embodiment 2: Preparation of 50mg / ml fulvestrant injection
[0028]
[0029]
[0030] Preparation: Weigh the prescribed amount of fulvestrant, ethanol (95%) and benzyl alcohol and mix, stir until fulvestrant is completely dissolved; add the prescribed amount of triglyceride caprylate-caprate to the above solution, stir, mix Evenly; add the prescribed amount of castor oil to the final volume (500ml), stir and mix. Filter the mixed solution through a 0.2 μm filter for 1 to 2 times to sterilize. In sterile, N-filled 2 Under protection, dispense samples into vials or prefilled syringes.
Embodiment 3
[0031] Embodiment 3: Preparation of 50mg / ml fulvestrant injection
[0032]
[0033] Preparation: Weigh the prescription amount of fulvestrant, ethanol (95%) and benzyl alcohol and mix them, stir until the fulvestrant is completely dissolved; add the prescription amount of glycerol triacetate to the above solution, stir, and mix well; add Add the prescribed amount of castor oil to the final volume (500ml), stir and mix. Filter the mixed solution through a 0.2 μm filter for 1 to 2 times to sterilize. In sterile, N-filled 2 Under protection, dispense samples into vials or prefilled syringes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com