Reverse thermal hydrogel preparations for use in the treatment of disorders of the urothelium
A technology of gel and gel-forming agent, which is applied in the directions of urological diseases, medical preparations without active ingredients, and medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Before testing Botulinum Toxin (BTX), it is added to Gel A as a treatment agent for bladder diseases, such as bladder spasm. The activity of botulinum in the gel and its release in the gel will be confirmed as follows:
[0088] The activity of BTX was determined by intramuscular injection into mice (Hsd: ICR female mice). Compared with the published method "Comparison of the effect of type A, B and F botulinum neurotoxin on the safety factor of mice" (Aoki , Korea, Journal of Toxicology, 2001, Issue 39, paragraphs 1815-1820), the above content is incorporated into the present invention by reference in its entirety. The commercially available BTX-A was dissolved in saline or gel, or released from the gel by saline to observe the effect of local muscle weakening (saline injection as a negative control group). The dose injected by the test animals was about 20 units / kg body weight, and the injection was injected into the right hind limb of each test animal with a dose volume...
Embodiment 2
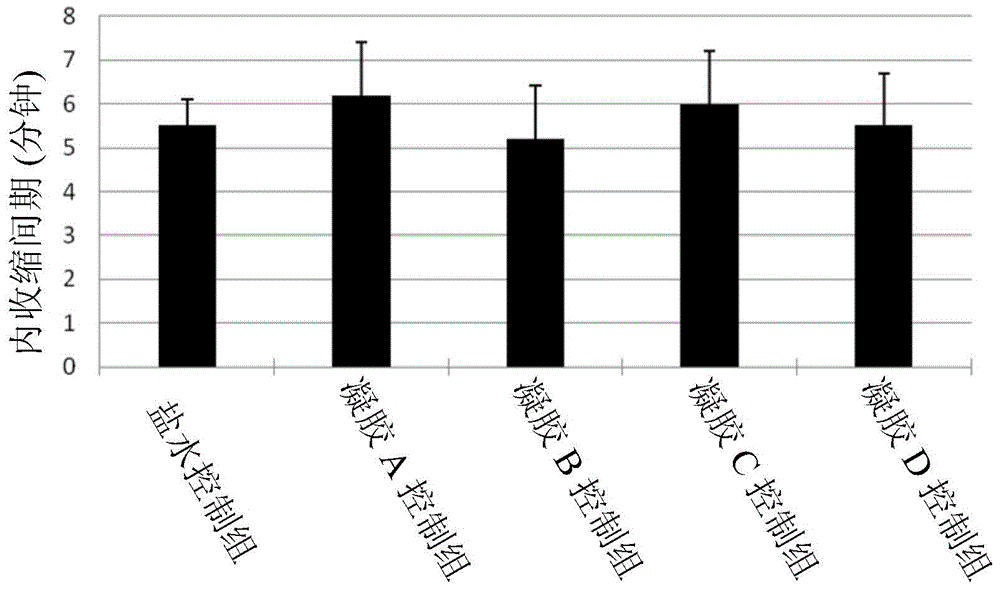
[0094] The following will combine the TheraCoat system for the treatment of bladder disease and observe the promoting effect of mixing for the treatment of bladder disease with a cystitis rat model. The test method is disclosed in "A type of botulinum toxin is administered to the bladder of rats to inhibit COX-2 and EP4 expression, and to inhibit the cyclophosphamide-induced cystitis of bladder hyperactivity" (Chuang, YC; Yoshimura, N.; Huang , CC; Wua, M. Chinese Journal of Urology, Issue 56, pages 159-167), the above content is incorporated into the present invention by reference in its entirety. Intraperitoneal injection of cyclophosphamide induces chronic cystitis (75 mg / kg injection on day 1, day 4 and day 7). On the second day, a polyethylene tube (PE-50) was inserted into the rat's bladder through the urethra. The bladder was emptied and instilled with and without BTX gel or saline (1 ml, 20 U / ml Allergan). On the 8th day, the animals were anesthetized, and the Miller ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com