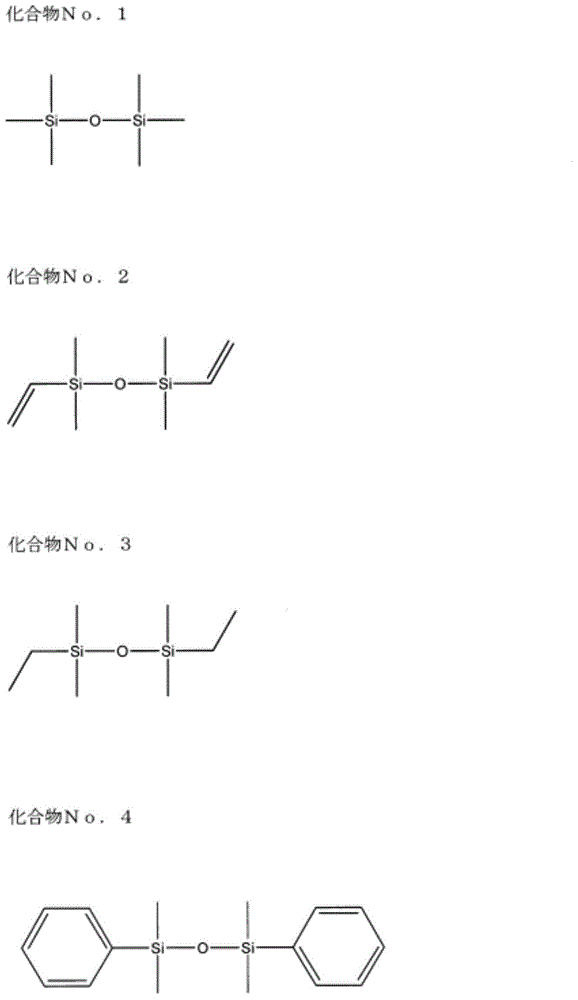
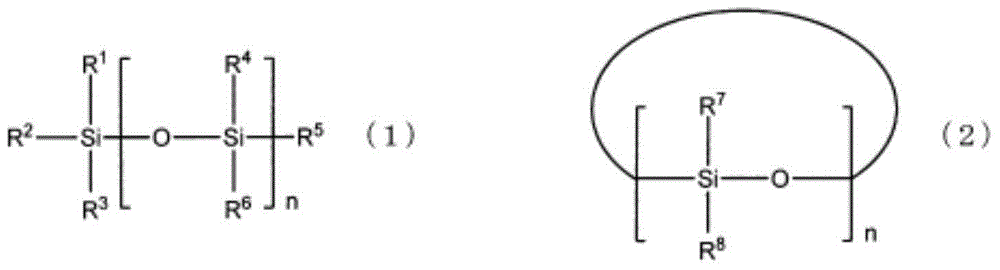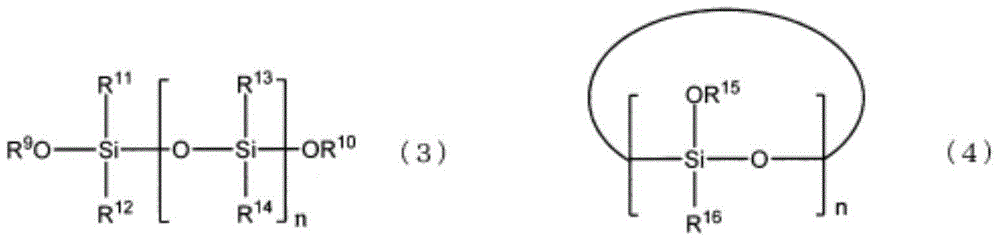Electrolyte for non-aqueous electrolyte battery, and non-aqueous electrolyte battery using same
A non-aqueous electrolyte and electrolyte technology, applied in non-aqueous electrolyte storage batteries, non-aqueous electrolytes, batteries, etc., can solve problems such as reduction in electric capacity, and achieve excellent low-temperature characteristics, improved residual rate, and excellent balance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0078] Use a mixed solvent of ethylene carbonate and ethyl methyl carbonate as a non-aqueous solvent at a volume ratio of 1:2, and dissolve LiPF as a solute in the solvent 6 1.0mol / L, 0.01% by mass of lithium difluorobis(oxalato)phosphate as the 1st compound, 11% by mass of the aforementioned compound No.11 as the 2nd compound, as shown in Table 1, prepare electrolytic solution for non-aqueous electrolyte battery liquid. It should be noted that the above preparation is carried out while maintaining the temperature of the electrolyte at 25°C. The free acid concentration in the electrolytic solution 1 hour after the above preparation was 54 mass ppm, and the free acid concentration in the electrolytic solution 24 hours after the preparation was 2 mass ppm. It should be noted that the measurement of free acid is carried out by titration.
[0079] Using this electrolyte, the LiCoO 2 Using graphite as the positive electrode material and graphite as the negative electrode materia...
Embodiment 1-2~1-37
[0097] The types and addition amounts of the first compound and the second compound in the aforementioned Example 1-1 were respectively changed to prepare an electrolyte solution for a non-aqueous electrolyte battery. Using this non-aqueous electrolytic solution, a battery cell was produced in the same manner as in Example 1-1, and battery evaluation was performed. Table 1 shows the preparation conditions of the nonaqueous electrolytic solution and the concentrations of free acids in the electrolytic solution after 1 hour and 24 hours after preparation, and Table 2 shows the evaluation results of batteries using the electrolytic solution. It should be noted that, compared with the battery cells of Examples 1-1 to 1-13, it was confirmed that the laminated battery cells of Examples 1-15 and 1-16 after the cycle test had larger swelling, and the gas inside the battery The production volume is relatively large. Compared with the battery cell of Comparative Example 1-1, the batter...
Embodiment 1-38~1-43
[0102] [Examples 1-38 to 1-43, Comparative Examples 1-7 to 1-10]
[0103] Change the negative pole body that uses among the embodiment 1-1, use non-aqueous electrolytic solution No.1-8,1-20,1-32,1-38 or 1-42 as non-aqueous electrolytic solution battery electrolytic solution, and implement In Example 1-1, the battery's initial capacity, cycle characteristics, internal resistance characteristics, and low-temperature characteristics were evaluated in the same manner. It should be noted that the active material in the negative electrode is Li 4 Ti 5 o 12 In Examples 1-38 to 1-40 and Comparative Examples 1-7 to 1-8, the negative electrode bodies were produced as follows: at 90% by mass Li 4 Ti 5 o 12 5% by mass of polyvinylidene fluoride (PVDF) as a binder and 5% by mass of acetylene black as a conductive agent were added to the powder, N-methylpyrrolidone was further added, and the resulting paste was applied on a copper foil. It was made to dry and it produced, and the end-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com