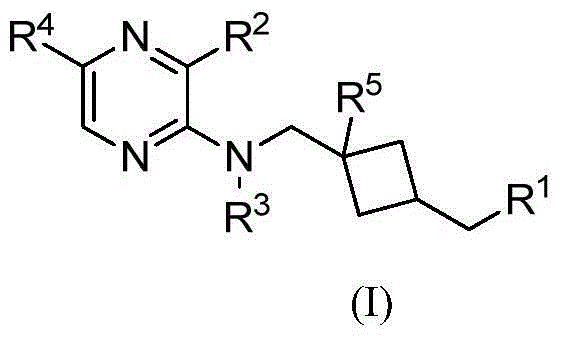
Pyrazine compound containing diethylene substituent and composition and application thereof
A kind of compound and composition technology, applied in the field of pyrazine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
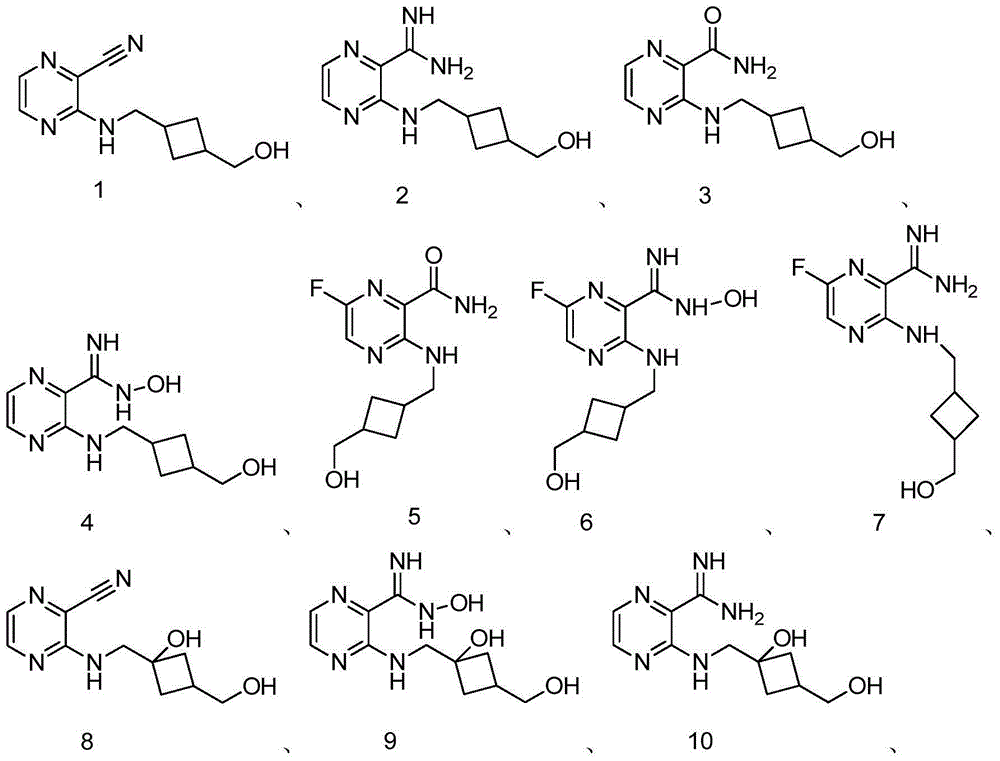
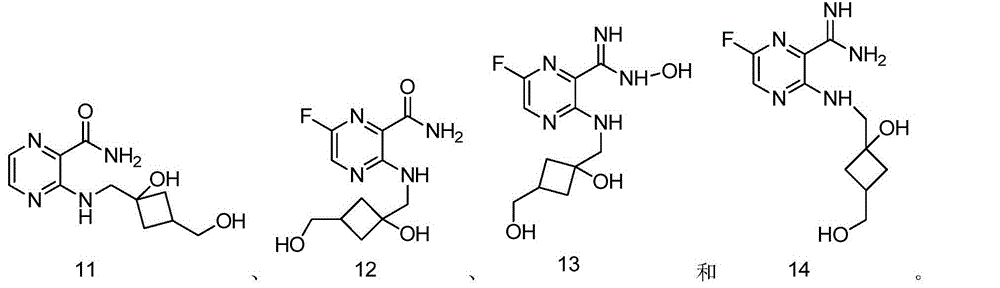
[0212] Example 1: 3-(((3-(hydroxymethyl)cyclobutyl)methyl)amino)pyrazine-2-carbonitrile
[0213]
[0214] Step 1: Synthesis of 3-chloropyrazine-2-carbonitrile
[0215] At 0°C, add phosphorus oxychloride (2 mL, 40 mmol) and diisopropylethylamine (3.5 mL) dropwise to a solution of 3-hydroxypyrazine-2-carboxamide (5 g, 37 mmol) in chlorobenzene (20 mL) ,80mmol). The reaction solution was stirred overnight at 90°C. After completion of the reaction, the reaction solution was concentrated under reduced pressure, the resulting residue was diluted with 20 mL of water, the resulting mixture was extracted with EtOAc (20 mL x 3), the combined organic phase was dried over anhydrous sodium sulfate, filtered and removed under reduced pressure, and the residue was filtered through a silica gel column. Purification by chromatography (eluent: PE / EtOAc (V / V) = 10 / 1) afforded the title compound (4.5 g, 79%) as a white solid.
[0216] 1 H NMR (400MHz, CD 3 OD):δ8.67(d,1H),8.63(d,1H).
[...
Embodiment 2
[0223] Example 2: 3-(((3-(hydroxymethyl)cyclobutyl)methyl)amino)pyrazine-2-carboxamidine
[0224]
[0225] Step 1: Synthesis of 3-((3-carbamimidinopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoate
[0226] Dissolve (3-((3-cyanopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoate (0.2 g, 3 mmol) in 10 mL of methanol, then add NaOMe (0.04 g, 6 mmol). The reaction solution was stirred at room temperature for 1 h, and NH 4Cl (0.03g, 5mmol), the resulting reaction solution was stirred overnight at room temperature. After the reaction, it was concentrated under reduced pressure, and the obtained residue was purified by silica gel column chromatography (eluent: DCM / MeOH=10 / 1) to obtain the title compound (0.12 g, 79%) as a white solid.
[0227] MS:(ESI,pos.ion)m / z:268.3[M+1] + .
[0228] Step 2: Synthesis of 3-((3-(hydroxymethyl)cyclobutyl)methylamino)pyrazine-2-carboxamidine
[0229] 3-((3-Carboxamidinopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoate (0.25 g, 4 mmol) was d...
Embodiment 3
[0231] Example 3: 3-(((3-(Hydroxymethyl)cyclobutyl)methyl)amino)pyrazine-2-carboxamide
[0232]
[0233] Step 1: Synthesis of (3-((3-carboxamidopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoate
[0234] Potassium carbonate (3.8mg, 0.02mmol, 0.1eq) was added to (3-((3-cyanopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoic acid (0.12g, 2mmol, 1eq ) in DMSO solution (10 mL), the mixture was stirred for 30 min, then 10 mL of hydrogen peroxide was added, the resulting mixture continued to react at room temperature for 2 h, and then 5 mL of water was added to quench the reaction, the solid product was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a residue, which was directly used in the next reaction .
[0235] MS:(ESI,pos.ion)m / z:268.3[M+1] + .
[0236] Step 2: Synthesis of 3-(((3-(hydroxymethyl)cyclobutyl)methyl)amino)pyrazine-2-carboxamide
[0237] Dissolve (3-((3-carboxamidopyrazin-2-ylamino)methyl)cyclobutyl)methylbenzoate (0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com