Freeze-drying excipient packing and transferring system and production method thereof
A technology of freeze-drying shaping and delivery system, which is applied in the direction of freeze-drying transportation, packaging, and packaged food, and can solve problems such as inaccurate dosage, assembly and use of pre-filled dual-chamber syringes, and impact on experience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
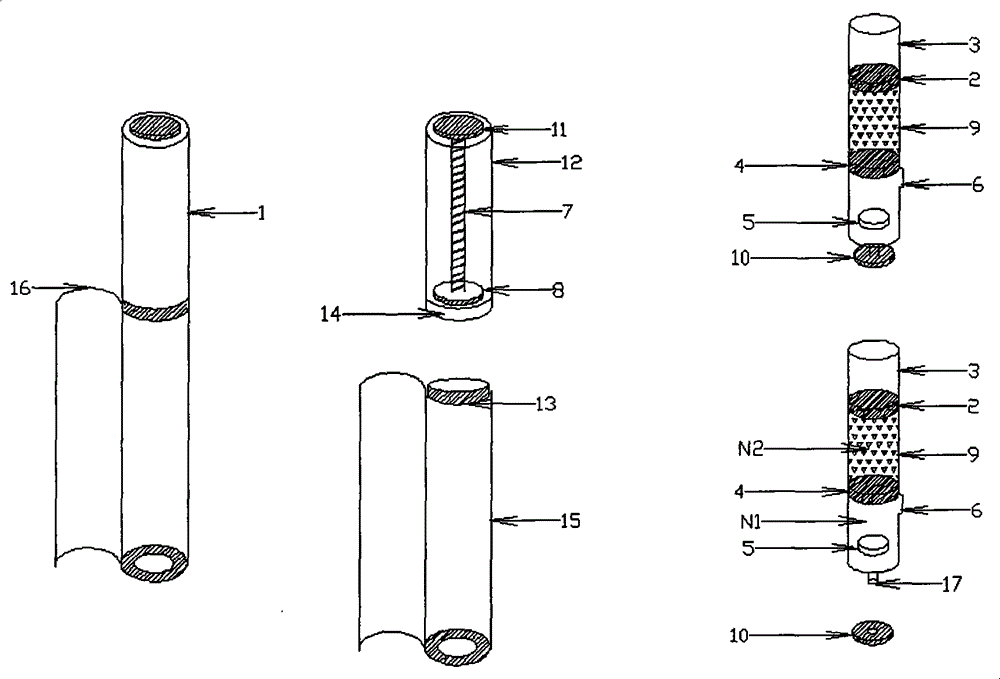
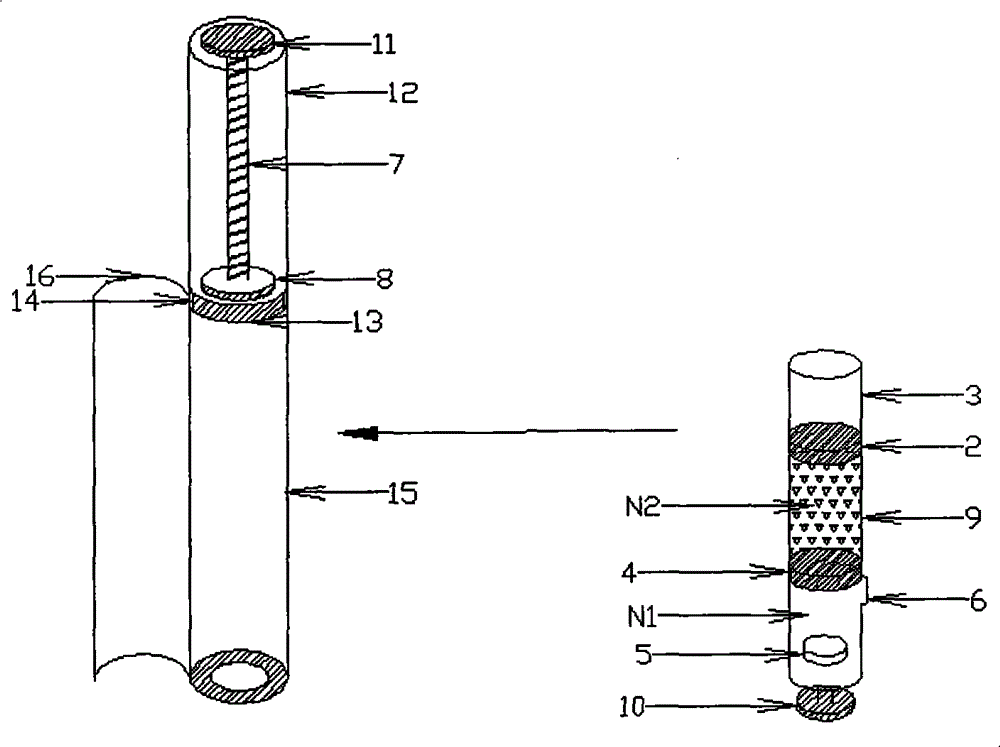
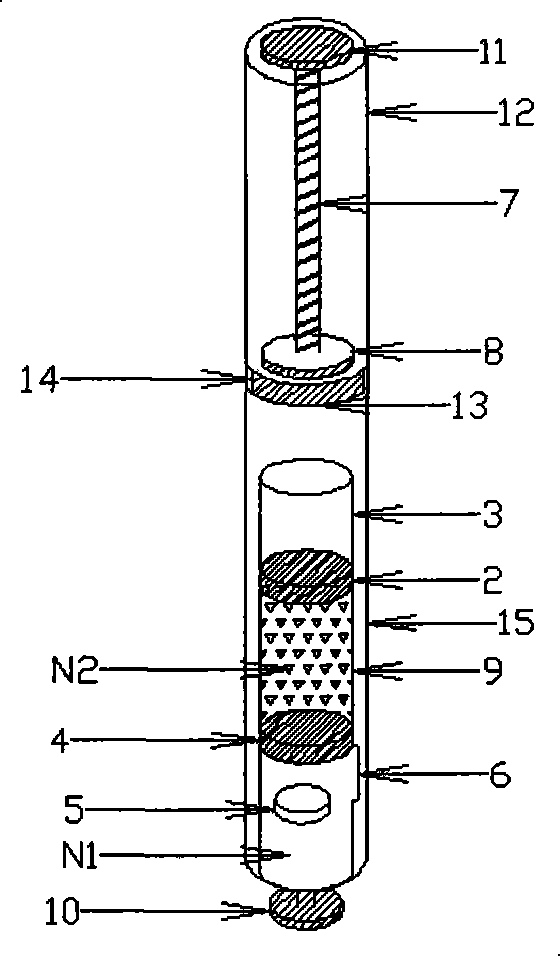
[0078] Vitamin C: Pullulan=5:1, 50 mg of the mixed powder is filled into a 0.4 milliliter molding mold in solid form, and then the remaining 10 mg is dissolved in 0.4 milliliters of water, filled into a molding mold containing the powder, and freeze-dried Finally, the obtained freeze-dried excipient preparation is loaded into the solid preparation cavity of the double-chamber mixing cavity of plastic material with a bypass 6, and then the piston 4 made of silica gel is assembled into the bottom of the solid preparation cavity of plastic material, Below the bypass 6; fill 2 milliliters of deionized water into the plastic liquid preparation chamber isolated by the piston 2 made of silica gel; then add the piston 2 made of silica gel, so that the liquid is completely stored in the liquid preparation chamber, and will not Seep out from between the silicone piston 4 and the silicone piston 2 to obtain a double-chamber mixing chamber containing a freeze-dried excipient and a solvent,...
Embodiment 2
[0082]EGF stock solution, add gelatin, hydrolyzed gelatin after thawing, be mixed with EGF (weight ratio) containing 5 / 100,000, gelatin+hydrolyzed gelatin solution containing 5%, fill into 0.1 milliliter molding mold, pack into after freeze-drying In the solid preparation chamber of a double-chamber transparent glass material with scale, then assemble the rubber piston 4 into the bottom of the solid preparation chamber and below the bypass 6; then fill 2 ml of 3% hyaluronic acid solution into the The liquid preparation cavity isolated by the rubber piston 2; then the rubber piston 2 is added, so that the liquid is completely stored in the liquid preparation cavity and will not seep from between the rubber piston 4 and the rubber piston 2, that is A double-chamber mixing chamber containing a freeze-dried excipient and a solvent is obtained, and a double-chamber mixer device is formed together with a transparent plastic accommodation chamber, a pushing chamber and a manual push r...
Embodiment 3
[0086] Panax notoginseng saponins: PVP=30mg: 15mg, prepared into a solution, filled into a 0.3ml molding mold, freeze-dried and packed into a double-chamber solid preparation chamber made of composite aluminum, and then the piston 4 made of silica gel was assembled into the The bottom of the solid preparation chamber, below the bypass 6; then fill 1 ml of deionized water containing natural essence into the liquid preparation chamber isolated by the piston 2 made of silica gel; then add the piston 2 made of silica gel to make the liquid complete Stored in the liquid preparation cavity, it will not seep from between the silicone piston 2 and the silicone piston 4, that is, a double-chamber mixing chamber containing freeze-dried excipients and solvents is obtained, and the container made of aluminum The cabin, the push cabin and the electric push rod piston together form a double-chamber mixer device.
[0087] When in use, put the electric push rod piston device 7, 8, 11 into the...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap