Preparation process of standard coprophilous fungi freeze-dried powder in transplantation therapy of coprophilous fungi
A preparation process, freeze-dried powder technology, applied in the medical field, can solve the problem of low activity of fecal bacteria freeze-dried powder, and achieve the effects of improving the survival rate of fecal bacteria, protecting stability, and counting accurately
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation process of standard fecal bacteria freeze-dried powder in the treatment of feces transplantation, comprising the following steps:
[0027] (1) Preparation of standard fecal liquid: 5 qualified donors (aged 25-35 years old, healthy males) were screened, 50 g of feces were collected aseptically, homogenized, filtered through gauze, centrifuged, and discarded. The supernatant liquid obtains the sludge sediment of fecal bacteria.
[0028] (2) Preparation of lyoprotectant: add 15% trehalose, 7% glycerin and 0.05% vitamin C to 100 mL sterile normal saline, mix well, and filter to sterilize.
[0029] (3) Three-step method of freezing and vacuum drying: add the freeze-drying protective agent into the fecal bacteria slurry, vortex and mix well, take 1 mL of the bacterial liquid for bifidobacteria culture and count, and put the remaining liquid into a freeze-drying tube. Then place the freeze-drying tube in the freeze-dryer and set the freeze-drying program: quickl...
Embodiment 1 5
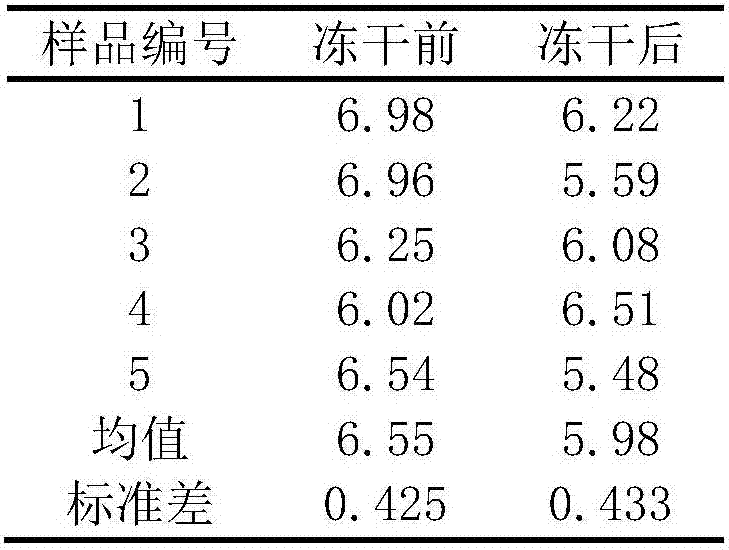
[0034] The fecal bacteria count result of five groups of parallel experiments of embodiment 1 is shown in Table 1:
[0035] Table 1 The total number of bifidobacteria ( lg10 n )
[0036]
[0037] The average total number of viable bifidobacteria in the sample before freeze-drying was 6.54±0.31log 10 cfu, the average number of viable bifidobacteria after three months of freeze-drying storage is 6.02±0.51log 10 cfu, the survival rate was 92%.
[0038] The result proves that the freeze-dried powder prepared by the process provided by the invention effectively maintains the activity of fecal bacteria and greatly improves the survival rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com