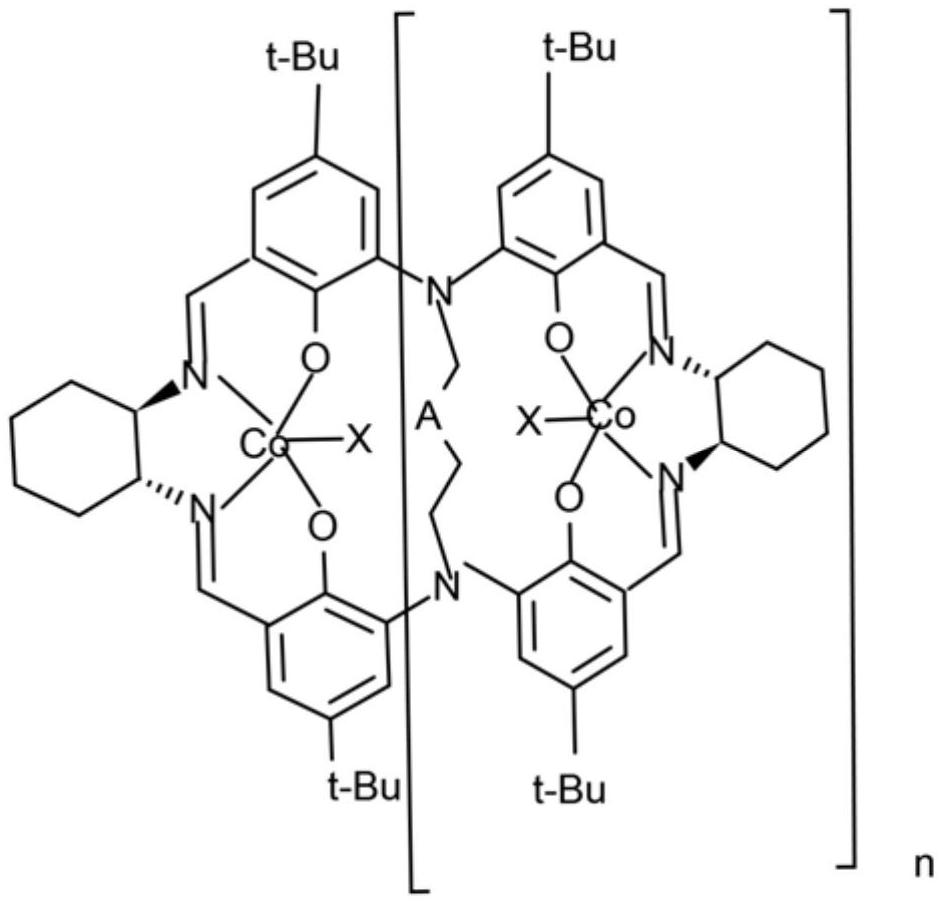
High-polymerization Salen cobalt catalyst as well as preparation method and application thereof
A cobalt catalyst, catalyst technology, applied in chemical instruments and methods, catalytic reactions, organic chemical methods and other directions, can solve problems such as unfavorable large-scale industrial application, complex catalyst synthesis, ester structure breakage, etc., and achieve large-scale industrial application. , high splitting efficiency, and the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
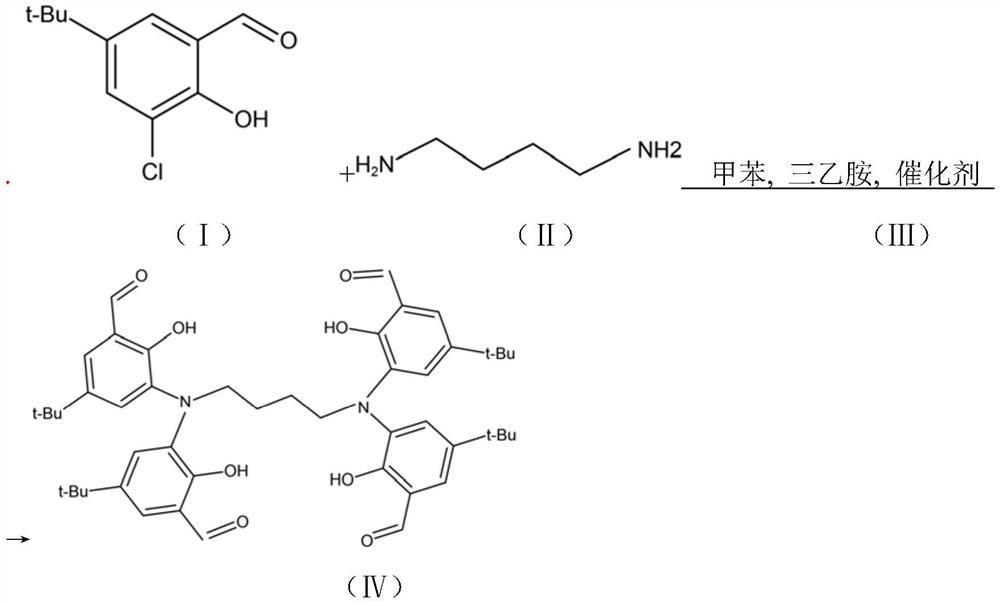
[0045] Embodiment 1 compound (Ⅳ) preparation
[0046]
[0047] Add 200mL of toluene to a 500ml four-necked flask with a stirring thermometer after drying; Add dropwise at 5°C: triethylamine (11.9g, 117.5mmol) and 1,4-butanediamine (2.27g, 25.8mmol) mixed solution, dropwise time 1-3 hours, after dropwise addition, 20- Keep warm at 30°C for 5h, slowly raise the temperature to 40-50°C and stir for 8h to stop the reaction. Wash twice with 100ml of deionized water to remove the catalyst tetrabutylammonium iodide and the triethylamine hydrochloride produced by the reaction, let stand to separate the layers, separate the organic layer, and the removed organic layer is dried with anhydrous sodium sulfate and suction filtered , After about half of the solvent was evaporated from the filtrate under reduced pressure, the temperature was lowered to 0° C., and light yellow crystals were precipitated. After suction filtration, vacuum drying gave 17.3 g of a light yellow solid (Compound ...
Embodiment 2
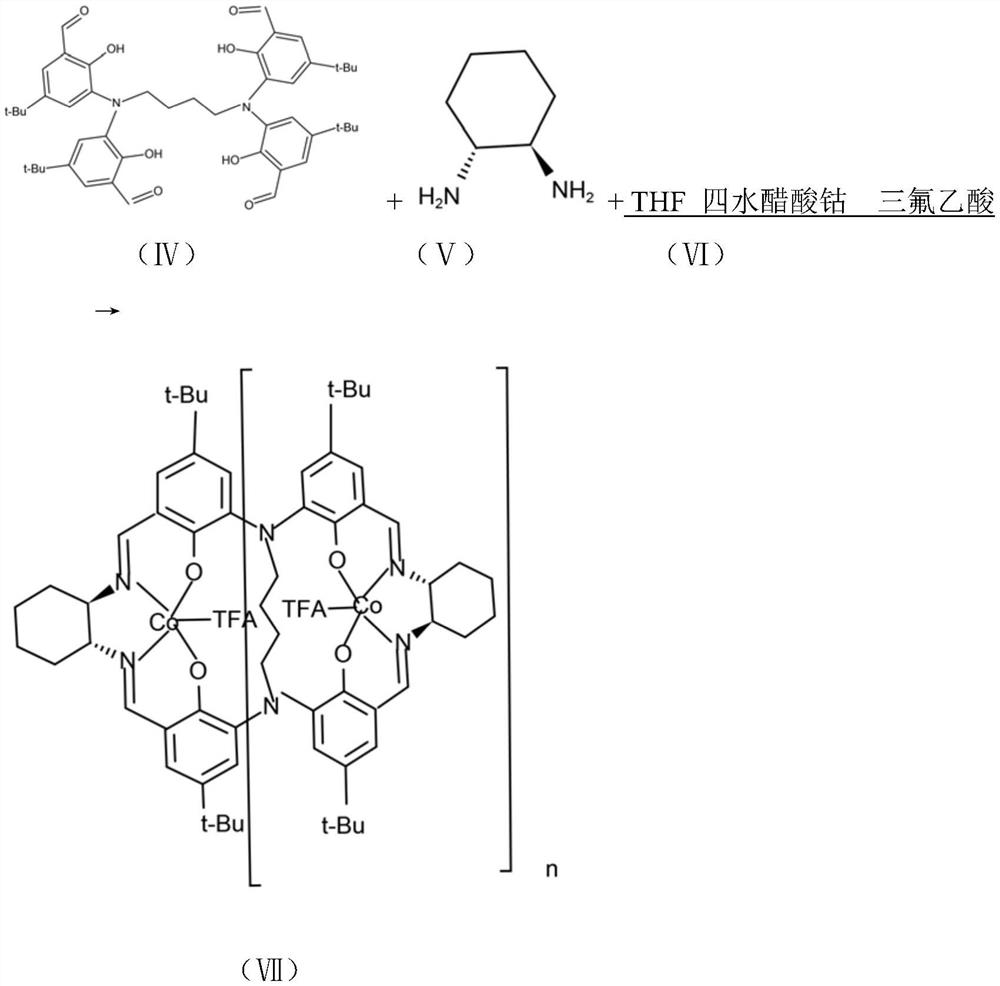
[0051] Preparation of Example 2 Compound VII
[0052]
[0053] After drying 500ml of a four-necked flask with a stirring thermometer, add 250mL of tetrahydrofuran; compound IV (20g, 25mmol), turn on the stirring water bath and add dropwise when the temperature rises to 60°C: (R,R)-1,2cyclohexanediamine (5.65g, 49.5mmol) and 10ml tetrahydrofuran to obtain a mixed solution. The dropwise addition time is 1 hour. After the dropwise addition is completed, stir at 65-70°C for 5h and cool down to 20°C. Add cobalt acetate tetrahydrate (12.4g, 50mmol) and stir at room temperature for 12h. Add trifluoroacetic acid (6g, 52.6mmol) dropwise and keep stirring at 10-20°C for 1 hour. Add 100ml of distilled water dropwise. During the dropwise addition, dark brown solids gradually precipitate. After the dropwise addition, filter with suction and vacuum dry to obtain dark brown 29 g of solid (compound VII), yield 91%.
[0054] MALDI-TOF MS(calcd for:C362H456O26N36CO12F36:7112.Found:7132[M+Na]...
Embodiment 3
[0055] Example 3 The application of compound (VII) in hydrolysis kinetic resolution of racemic terminal epoxy compound to obtain chiral terminal epoxy compound and anti-configuration chiral diol:
[0056]
[0057] After drying 1000ml of four-necked flask with stirring thermometer, add epichlorohydrin (600g, 6496mmol), turn on the stirring ice bath and cool to 15 degrees, add compound (Ⅶ) (0.78g, 0.6mmol), dropwise add deionized water (67.2g , 3735mmol) temperature 10-20 degrees; drop time 2-3 hours. After the dropwise addition, keep warm at 20°C for 12 hours, take a sample of about 2 μL, dilute it with dichloromethane, measure the reaction progress with chiral gas chromatography (GC), and stop the reaction when the optical purity of S epichlorohydrin in the sample reaches 99.5% ee . Low-boiling S-epichlorohydrin was collected by vacuum distillation to obtain high-boiling R-3-chloro-1,2-propanediol. After the distillation was completed, the temperature was lowered to 20°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com