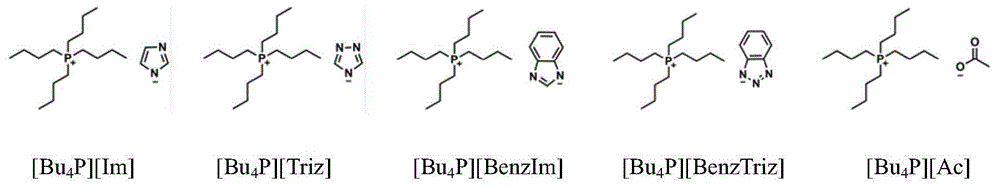
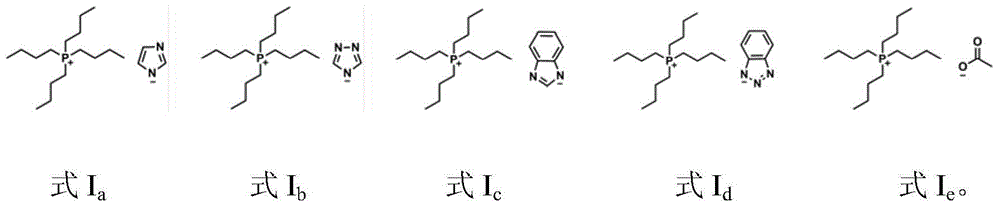
Catalytic system for synthesizing alpha-hydroxy ketone by alkynol hydration reaction
A catalytic system, acetylenic alcohol hydration technology, applied in the direction of organic compound/hydride/coordination complex catalyst, carbon-carbon triple bond hydration preparation, physical/chemical process catalyst, etc., can solve rearrangement and other problems, and achieve high Catalytic activity, easy synthesis and recovery, wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Catalyzing the hydration reaction of 2-methyl-3-butyn-2-ol to generate 3-hydroxyl-3-methyl-2-butanone
[0024] In a 250 ml single-necked bottle, 2-methyl-3-butyn-2-ol (8.41 g, 0.1 mol), H 2 O (3.60g, 0.2mol) and formula I a [Bu 4 P][Im] (97.89g, 0.3mol), with CO 2 displace the air therein; then keep the CO 2 The pressure was 0.1MPa, refluxed and stirred at 80°C for 24 hours. After the reaction is finished, with tert-butanol as internal standard, by 1 H NMR quantitative detection, using the conventional internal standard method for calculation, the yield of 3-hydroxy-3-methyl-2-butanone was 90%.
[0025] reaction product 1 H and 13 C nuclear magnetic spectrum to determine its structure:
[0026] 1 H NMR (DMSO-d6, 400 MHz) δ 5.21 (s, 1H), 2.15 (s, 3H), 1.17 (s, 6H).
[0027] 13 C NMR (DMSO-d 6 , 100MHz) δ213.64, 75.76, 26.15, 24.11.
[0028] It can be seen from the above that the product has a correct structure and is 3-hydroxy-3-methyl-2-butanone....
Embodiment 2
[0029] Example 2, catalyzing the hydration reaction of 2-methyl-3-butyn-2-ol to generate 3-hydroxyl-3-methyl-2-butanone
[0030] Adopt exactly the same reaction condition and detection method as embodiment 1, only [Bu 4 The amount of P][Im] was changed to 163.15g (0.5mol), and the yield of 3-hydroxyl-3-methyl-2-butanone was 88%.
Embodiment 3
[0031] Example 3, catalyzing the hydration reaction of 2-methyl-3-butyn-2-ol to generate 3-hydroxy-3-methyl-2-butanone
[0032] Adopt exactly the same reaction condition and detection method as embodiment 1, only [Bu 4 P][Im] amount is changed into 65.26g (0.2mol), CO 2 The gas pressure was changed to 1 MPa, the reaction time was changed to 24 h, and the yield of 3-hydroxy-3-methyl-2-butanone was 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com