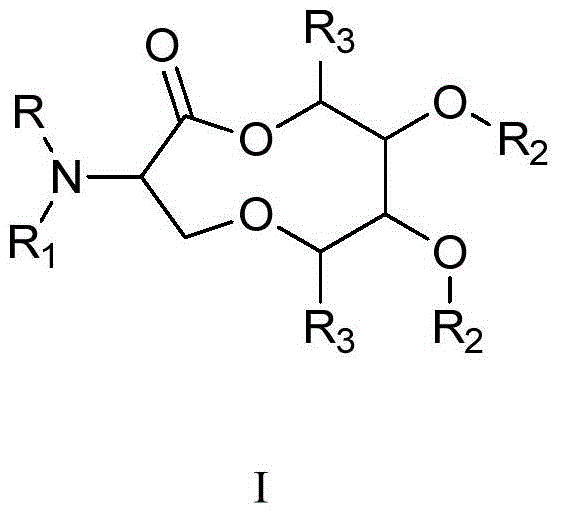
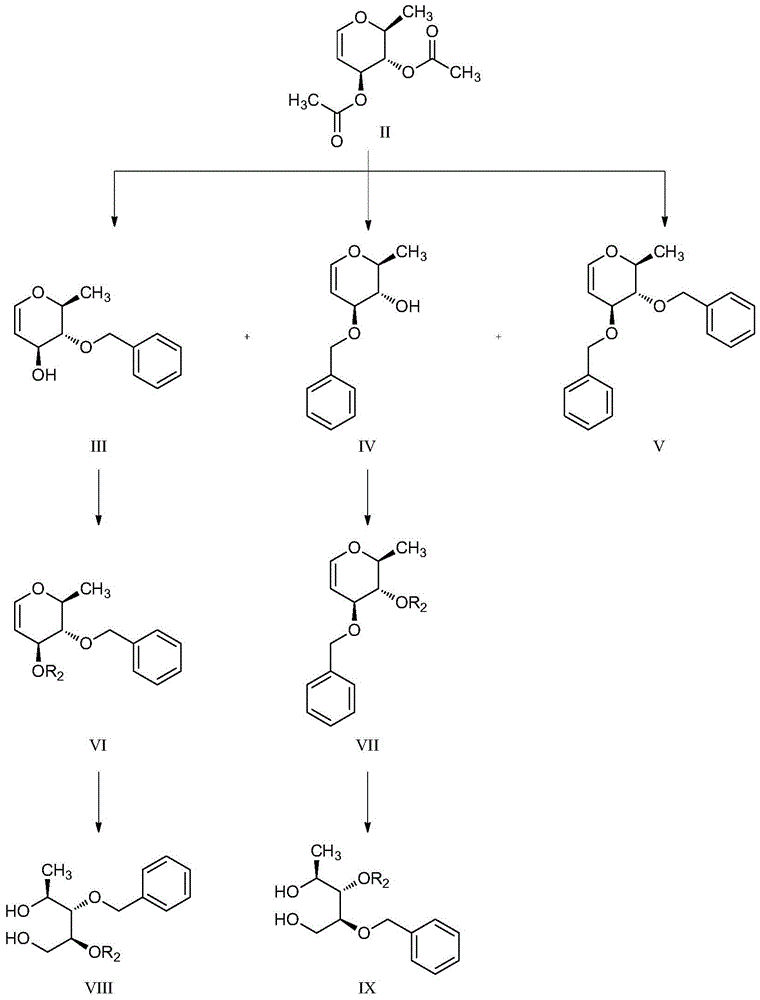
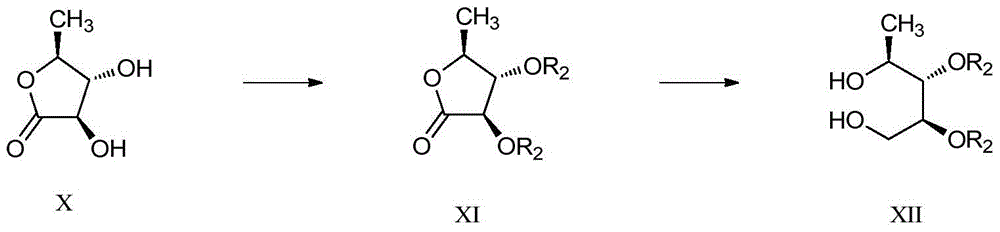
Macrocyclic pyridine‑2‑amides as fungicides
A technology of fungi and pesticides, applied in the direction of fungicides, biocides, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1, Step 1: Preparation of (3R,4S,5S)-5-methyl-3,4-bis(2-methallyloxy)dihydrofuran-2(3H)-one
[0104]
[0105] In nitrogen (N 2 ), to stirred (3R,4R,5S)-3,4-dihydroxy-5-methyldihydrofuran-2(3H)-one (1.3 grams (g), 9.84 millimoles (mmol)) and Pd(Ph 3 P) 4 (0.569 g, 0.492 mmol) in anhydrous THF (9 milliliters (mL)) was added bis(2-methallyl) carbonate. Stir the solution at 60°C for 1 hour (h) (CO 2 After evolution ceased), the reaction mixture was cooled and the solvent was evaporated. By flash chromatography (silica gel (SiO 2 )) Purification of the crude product afforded the title compound as a colorless oil (2.3 g, 97%): 1 H NMR (400MHz, CDCl 3 )δ5.04(dd,J=1.9,0.9Hz,1H),5.00-4.98(m,1H),4.97-4.93(m,2H),4.47-4.38(m,1H),4.36-4.27(m, 1H), 4.23-4.00(m, 4H), 3.82(t, J=7.2Hz, 1H), 1.78(s, 3H), 1.75(s, 3H), 1.48(d, J=6.3Hz, 3H); 13 C NMR (101MHz, CDCl 3 )δ172.65,141.07,140.92,113.54,113.14,84.19,79.31,76.57,74.53,74.51,19.48,19.35,19.00; EIMS m / z 240[M + ]...
Embodiment 2
[0127] Example 2, step 1: (S)-2-(benzyloxycarbonylamino)-3-((2S,3S)-2,3-bis(benzyloxy)-4-hydroxybutoxy) Preparation of methyl propionate:
[0128]
[0129] At -78°C, to (2S,3S)-2,3-bis(benzyloxy)butane-1,4-diol (283mg, 0.935mmol) (such as Journal of Organic Chemistry 1991,56(3) , prepared as described in 1321-1322) and (S)-aziridine-1,2-dicarboxylate 1-benzyl 2-methyl ester (110 mg, 0.468 mmol) in CH 2 Cl 2 (1.5 mL) was added BF 3 -OEt 2 (10 μL, 0.081 mmol), and the reaction mixture was stirred at -78 °C for 1 h, slowly warmed to 0 °C, then stirred at 0 °C for 3 h. By flash chromatography (SiO 2 ) directly purified the reaction mixture to afford the title compound as a colorless oil (208 mg, 83%): 1 HNMR (400MHz, CDCl 3 )δ7.45-7.20(m,16H),5.70(d,J=8.5Hz,1H),4.70-4.54(m,6H),4.53-4.46(m,1H),3.93(dd,J=9.5, 3.2Hz,1H),3.80-3.53(m,9H),2.09-1.96(m,1H); 13 C NMR (101MHz, CDCl 3 )δ170.72,156.04,138.16,138.08,136.24,128.54,128.49,128.47,128.20,128.13,127.99,127.91,127.89,12...
Embodiment 3
[0143] Example 3, Step 1: Preparation of (2R,3R)-2,3-di((2-methallyl)oxy)dimethyl succinate:
[0144]
[0145] To a cooled (-10 °C) suspension of NaH (60% dispersion in mineral oil, 6.73 g, 168 mmol) in DMF (240 mL) was added dropwise (+)-dimethyltartrate (+)-L-tartrate over 30 min. 15.0 g, 84.0 mmol) in DMF (40 mL). After the addition was complete, the mixture was stirred at -10 °C for an additional 30 min, then 3-bromo-2-methylpropene (25.5 mL, 253 mmol) was added while keeping the reaction temperature below 0 °C. The mixture was stirred at -10 °C for 1.5 h, washed with H 2 It was quenched with O and saturated aqueous sodium chloride (NaCl, brine), and extracted with EtOAc. The two phases were separated and the aqueous phase was further extracted with EtOAc. The combined organic phases were washed with brine, washed with magnesium sulfate (MgSO 4 ) was dried, filtered and concentrated to give a yellow oil. By flash chromatography (SiO 2 , hexane / EtOAc gradient) to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com