Arterial hemostasisdressing and preparation method
A hemostatic dressing and arterial technology, applied in the field of medical devices, can solve problems such as changes in hemostatic properties, insignificant effects, and inability to stop bleeding at the rescue site, achieving high biological safety and avoiding ischemic necrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method for an arterial hemostatic dressing, comprising the steps of:
[0038] (1) Calcium alginate fibers are cut into 50mm long calcium alginate staple fibers, and chitosan fibers with a deacetylation degree of more than 95% and a molecular weight of 200,000-300,000 Daltons are cut into 50mm long chitosan short fibers. Fiber;
[0039] (2) According to the ratio of mass ratio of 6.5:3.5, calcium alginate short fiber and chitosan short fiber are evenly mixed and then carded by a carding machine. It is made into a thickness of 4mm by acupuncture and a density of 0.18g / cm 3 Calcium alginate chitosan composite non-woven fabric;
[0040] (3) 3mm thick, density 0.14g / cm 3 Calcium alginate non-woven layer and 4mm thick with a density of 0.18g / cm 3 Calcium alginate-chitosan composite non-woven fabric layers are stacked and laminated by acupuncture to obtain an arterial hemostatic dressing.
Embodiment 2
[0042] A preparation method for an arterial hemostatic dressing, comprising the steps of:
[0043] (1) Calcium alginate fibers are cut into 40mm long calcium alginate short fibers, and the chitosan fibers with a deacetylation degree of more than 95% and a molecular weight of 200,000-300,000 Daltons are cut into 40mm long chitosan short fibers. Fiber;
[0044] (2) According to the mass ratio of 6:4, calcium alginate short fiber and chitosan short fiber are evenly mixed and then combed by a carding machine. It is made into a thickness of 3mm by acupuncture and a density of 0.14g / cm 3 Calcium alginate chitosan composite non-woven fabric;
[0045] (3) 3mm thick, density 0.14g / cm 3 Calcium alginate non-woven layer and 3mm thick with a density of 0.14g / cm 3 Calcium alginate-chitosan composite non-woven fabric layers are stacked and laminated by acupuncture to obtain an arterial hemostatic dressing.
Embodiment 3
[0047] A preparation method for an arterial hemostatic dressing, comprising the steps of:
[0048] (1) The calcium alginate fiber is cut into 60mm long calcium alginate short fiber, and the chitosan fiber with a deacetylation degree of more than 95% and a molecular weight of 200,000-300,000 Daltons is cut into a 60mm long chitosan short fiber. Fiber;
[0049](2) According to the ratio of mass ratio of 7:3, calcium alginate short fiber and chitosan short fiber are evenly mixed and then carded by a carding machine. It is made into a thickness of 4mm by acupuncture and a density of 0.18g / cm 3 Calcium alginate chitosan composite non-woven fabric;
[0050] (3) 4mm thick, density 0.18g / cm 3 Calcium alginate non-woven layer and 4mm thick with a density of 0.18g / cm 3 Calcium alginate-chitosan composite non-woven fabric layers are stacked and laminated by acupuncture to obtain an arterial hemostatic dressing.
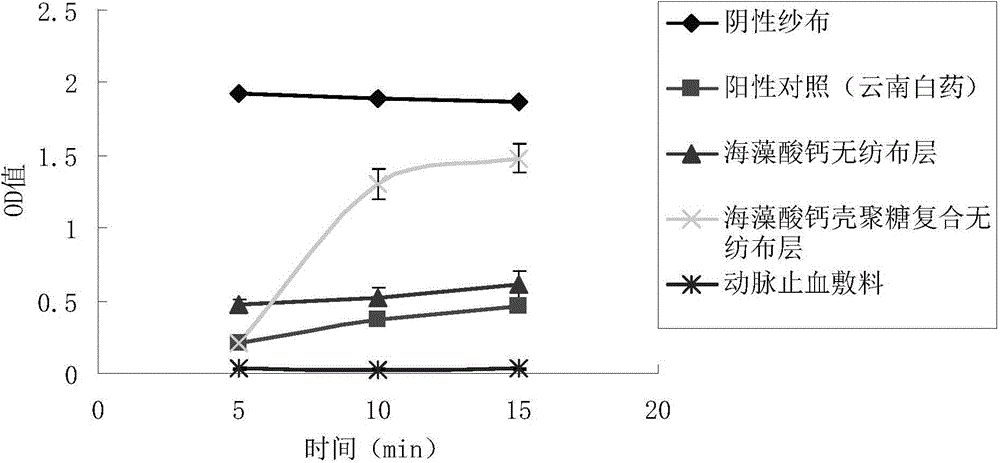
[0051] When using the product of the present invention, the calcium alg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com