Adamantane amides derivative and preparation method and application thereof
A technology of alkyl compounds, applied in the field of drugs for the treatment of diabetes, can solve problems such as undiscovered weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
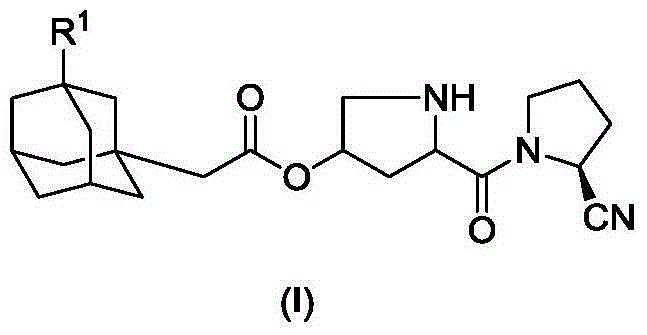
[0027]
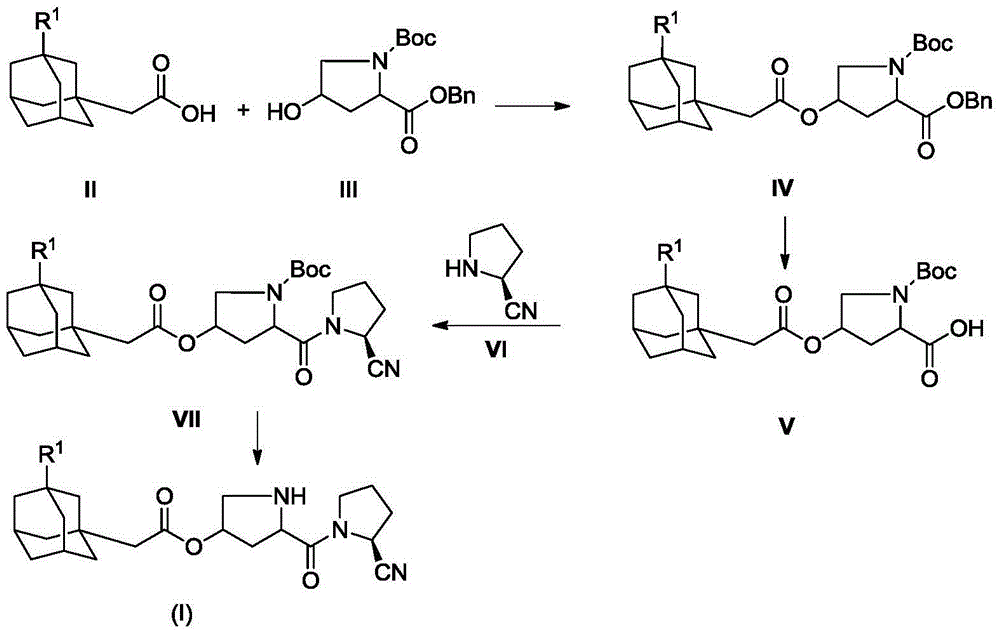
[0028] In a 100mL round bottom flask, add 1.94g (10mmol) compound II-1, 3.21g (10mmol) compound III, 2.06g (10mmol) N, N'-dicyclohexylcarbodiimide (DCC) and 1.22g (10mmol) 4-dimethylaminopyridine (DMAP), dissolved in 20mL of dry THF, stirred at room temperature overnight, TLC showed that the reaction was almost complete. The reaction mixture was suction filtered to remove the solid, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by column chromatography to obtain compound IV-1, a white solid, ESI-MS, m / z=515 ([M+NH 4 ] + ).
[0029] 2.99g (6mmol) of compound IV-1 was dissolved in 30mL of THF, 0.10g of 10% Pd / C was added, catalytic hydrogenolysis was carried out at room temperature, and the reaction was completed within 12 hours. The reaction mixture was suction filtered to remove the catalyst, and the filtrate was concentrated on a rotary evaporator, poured into 200 mL of water, stirred, and extracted with 50 mL×3 dich...
Embodiment 2
[0033]
[0034] In a 100mL round bottom flask, add 2.08g (10mmol) compound II-2, 3.21g (10mmol) compound III, 2.06g (10mmol) N, N'-dicyclohexylcarbodiimide (DCC) and 1.22g (10mmol) 4-dimethylaminopyridine (DMAP), dissolved in 20mL of dry THF, stirred at room temperature overnight, TLC showed that the reaction was almost complete. The reaction mixture was suction filtered to remove the solid, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by column chromatography to obtain compound IV-2 as a white solid, ESI-MS, m / z=529 ([M+NH 4 ] + ).
[0035] 3.07g (6mmol) compound IV-2 was dissolved in 30mL THF, 0.10g 10% Pd / C was added, catalytic hydrogenolysis was carried out at room temperature, and the reaction was completed within 12 hours. The reaction mixture was suction filtered to remove the catalyst, and the filtrate was concentrated on a rotary evaporator, poured into 200 mL of water, stirred, and extracted with 50 mL×3 dichloromet...
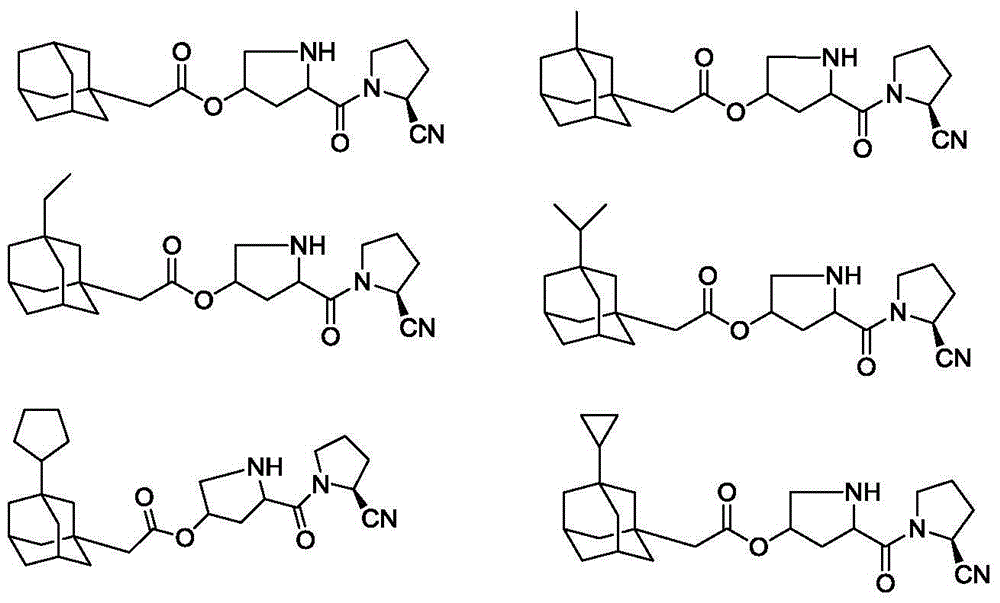
Embodiment 3-6
[0039] Using the same method as in Example 1, the following compounds could be prepared.
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com