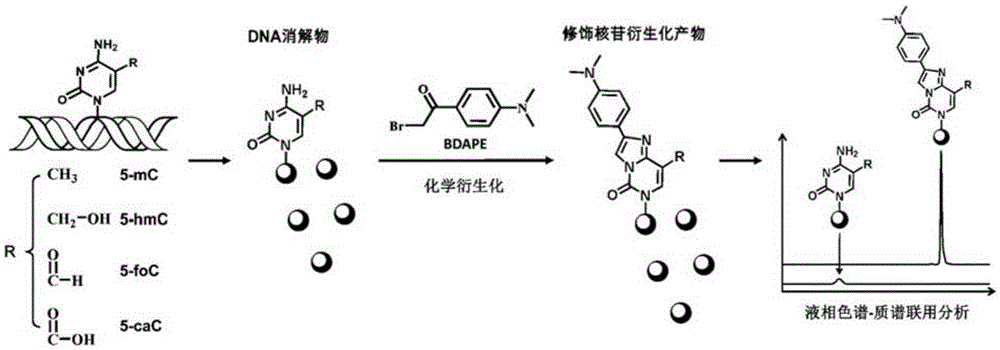
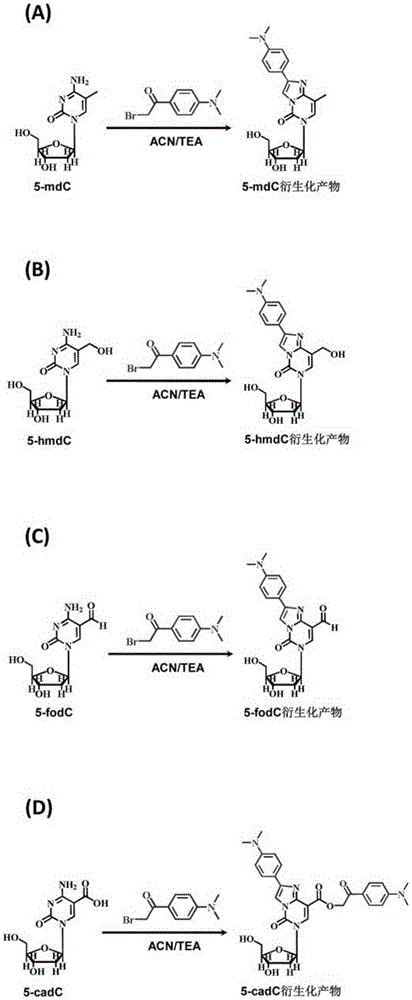
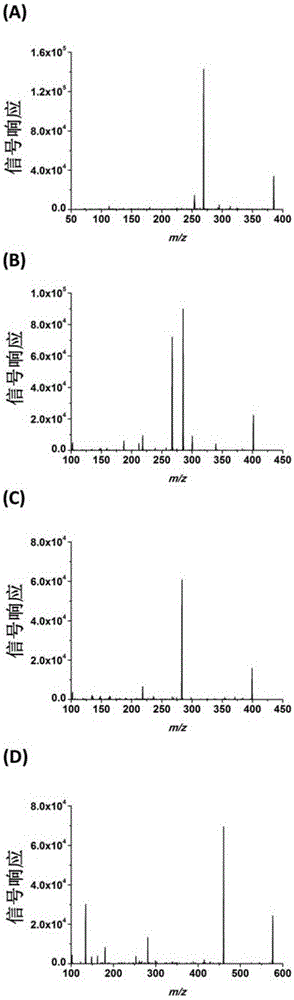
A chemical derivatization method and its application in detection of nucleic acid modification by lc-ms method
A derivatization and chemical technology, applied in the direction of biochemical equipment and methods, microbial determination/inspection, measuring devices, etc., can solve the problems of cumbersome operation, time-consuming and labor-intensive, insufficient detection sensitivity, etc., to avoid contamination of mass spectrometer, broad Application prospect, beneficial effect of quantitative analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Human Cell DNA Analysis
[0045] Three human cell lines: HeLa (cervical carcinoma cells), Jurkat-T (leukemic lymphocytes) and 293T (human embryonic kidney cells). Take a certain number of cultured cells and wash them with PBS buffer, put them in a 1.5mL centrifuge tube, centrifuge at 2000g for 20s to pellet the cells, and discard the supernatant. Add 0.5mL cell lysate A (320mM sucrose, 5mMMgCl 2 , 10mM Tris, 0.1mM deferoxamine, pH 7.5, 1% TritonX-100), shake vigorously with a micro-vortex mixer for 30s. The sample was centrifuged at 16000g for 20s, the supernatant was discarded, and the previous process was repeated. Then add 0.2 mL of cell lysate B (10 mM Tris, 5 mM EDTA, 0.15 mM deferoxamine, pH 8.0, 1% sodium lauryl sarcosine), and shake vigorously for 30 s with a micro-vortex mixer. Add 10 μL RNaseA (1 mg / mL) and 3 μL RNaseT1 (1 U / μL), and incubate in a water bath at 50° C. for 15 minutes to remove RNA. Then add 10 μL proteinase K (20mg / mlinH 2 O), i...
Embodiment 2
[0049] Example 2: DNA Analysis of Cancer Tissues and Paracancerous Tissues of Colorectal Cancer Patients
[0050] Take formalin-fixed, paraffin-embedded colorectal patient tissue sections (5-10 slices, about 30 mg), and use a paraffin-embedded tissue DNA extraction kit ( FFPEDNAKit, OmegaCo.) extracts DNA from tissues. The extracted DNA was dissolved in water and quantified by an ultra-micro ultraviolet spectrophotometer. Take 2-20 μg DNA, dry it in vacuum, add 17 μL water and 2 μL S1 nuclease buffer (300 mM sodium acetate, pH 4.6, 2800 mM sodium chloride, 10 mM zinc sulfate) in turn, place in 95 ° C water bath for 5 minutes, and then quickly place in Quenching in an ice-water bath for 2 minutes to untangle the DNA double strands, add 1 μL S1 nuclease (180 U / μL) and incubate in a 37° C. water bath for 12-16 hours. Then add 65 μL water, 10 μL alkaline phosphatase buffer (500 mM Tris-HCl, 100 mM magnesium chloride, pH 9.0), 1 μL alkaline phosphatase (30 U / μL), 4 μL snake veno...
Embodiment 3
[0053] Example 3: Yeast Cell DNA Analysis
[0054]Ten yeast strains: Saccharomyces cerevisiae BY4741, Saccharomyces cerevisiae W1588-4C, Debaryomyceshansenii, Schizosaccharomycespombe, Kluyveromyces marxianus , Schizosaccharomycesoctosporus, Yarrowialipolytica, Pichia pastoris, Saccharomyces boulardii and Kluyveromyceslactis were cultured, and the yeast cells The suspension was centrifuged at 4600g for 5 minutes, the collected cell pellet was washed with water, and the residual medium was removed. Disperse the yeast cells in 1 mL of cell lysate (10 mM Tris-HCl, pH8.0, 1 mM EDTA, 100 mM NaCl, 10 g / LSDS, 20 g / L TritonX-100), and then add an equal volume of phenol / chloroform (1:1, v / v , phenol saturated with 10mM Tris, 1mM EDTA, pH 8) and 1.5g glass beads (425-600μm), shake vigorously with a micro-vortex mixer for 10min. Then 1 mL LTE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) was added to the solution, and centrifuged at 13500 g for 5 minutes. Transfer the upper aqueous phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com