Method for simultaneously determining tryptophan, kynurenine and kynurenicacid by using high performance liquid chromatography-fluorescence method
A high-performance liquid chromatography and kynurenic acid technology, applied in the field of analytical chemistry, can solve the problems of cumbersome and time-consuming experimental operations, and achieve the effects of simple operation, true and accurate determination, and rapid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Establishment of chromatographic conditions and preparation of standard solutions
[0038] Preparation of standard solution
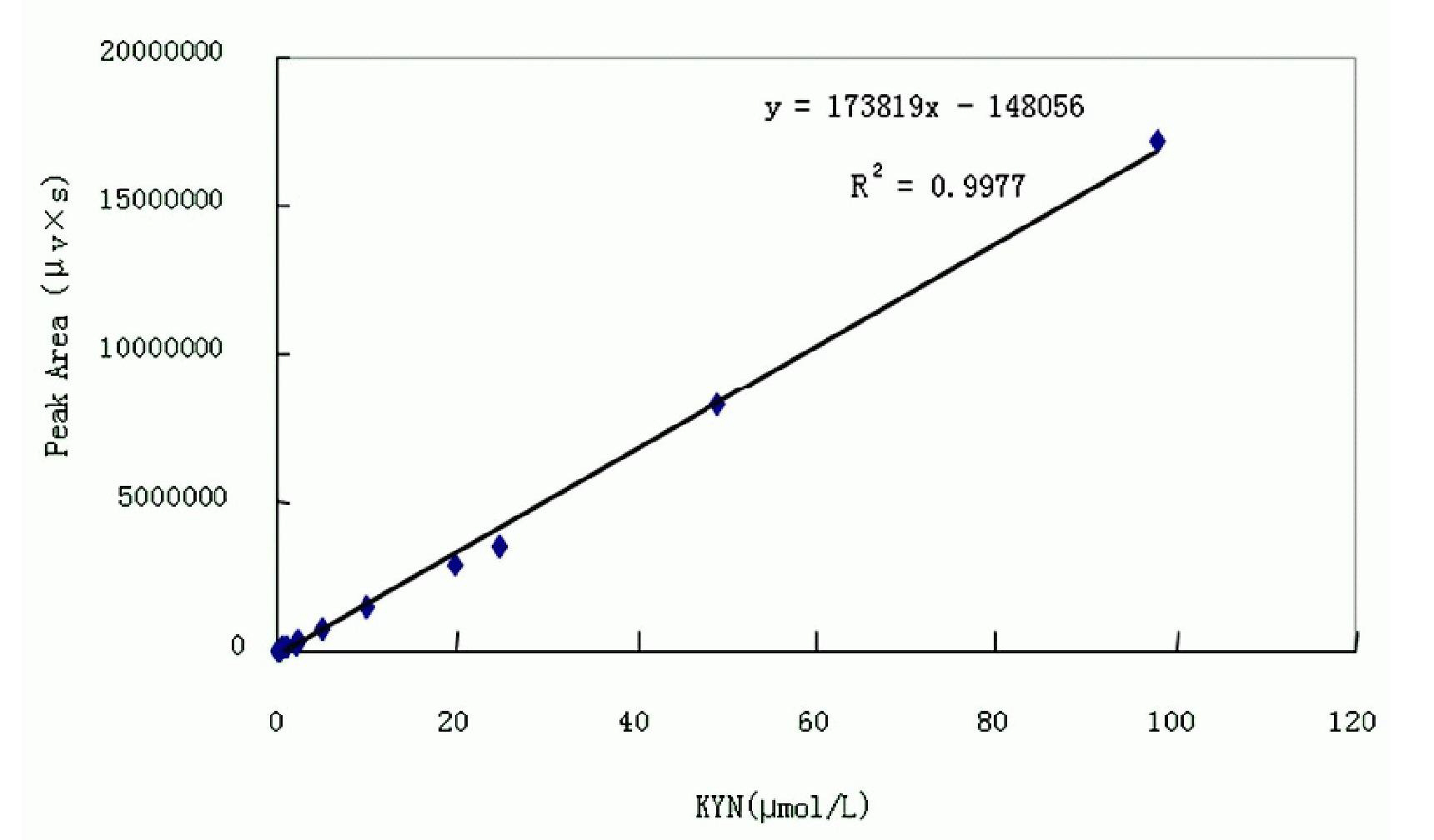
[0039] Accurately weigh Trp100.0mg, Kyn108.0mg and Kyna99.0mg, dissolve and dilute to 100ml with 0.312mol / L perchloric acid solution, mix well, and make 4900μmol / L Trp, 1960μmol / L Kyn and 104.67μmol / L respectively Standard stock solution of L Kyna, aliquoted and stored in a -20°C refrigerator for later use. Before use, take the standard stock solution and add ultrapure water to make a mixed standard solution (containing Kyn 0.98μmol / L, Kyna 26.17nmol / L and Trp 24.5μmol / L).
[0040] Second, the choice of high performance liquid chromatography conditions
[0041] 1. Selection of detection wavelength
[0042] Using the manual stop-flow technique, the maximum excitation light wavelength is obtained by scanning the excitation light spectrum (range 200-450nm) and emission light spectrum (range 300-550nm) of Kyn, Kyna and Trp respectively: λex Tr...
Embodiment 2
[0054] Sample testing
[0055] 1. Detection of mixed standard solution
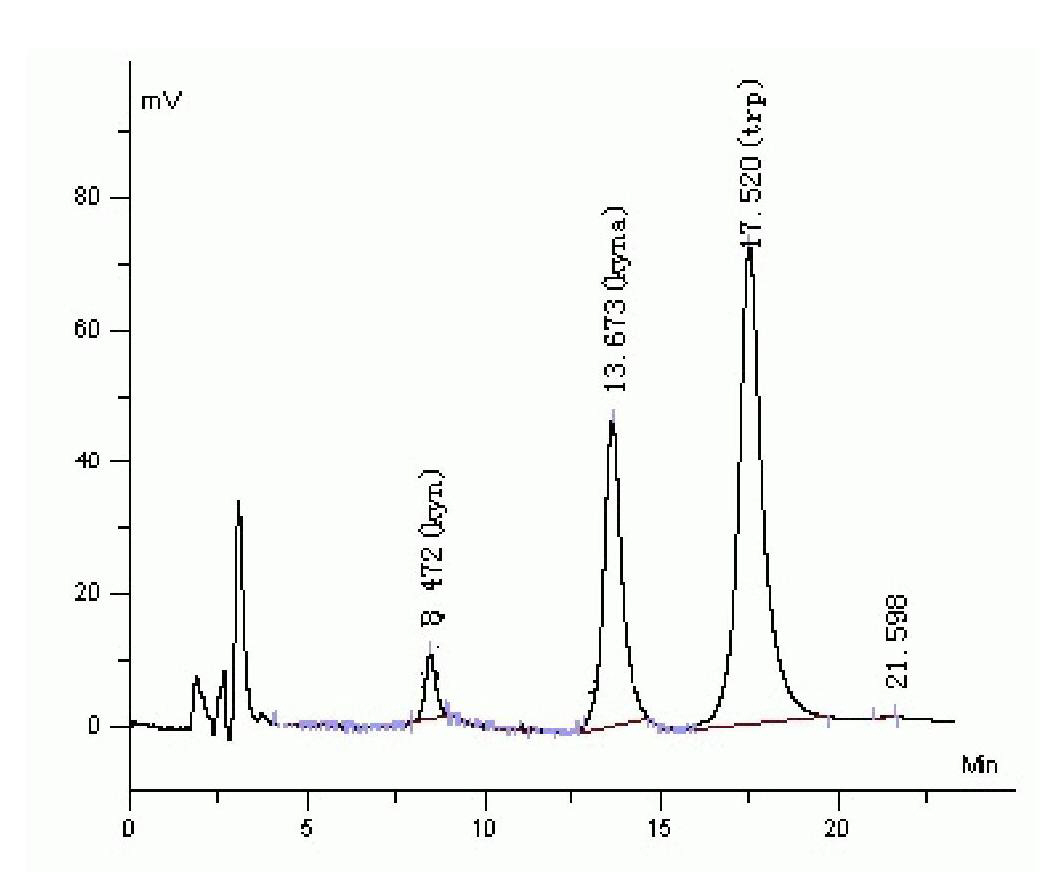
[0056] Take the standard stock solution prepared above and add ultrapure water to make a mixed standard solution (containing Kyn 0.98 μmol / L, Kyna 26.17nmol / L and Trp 24.5 μmol / L) for analysis. High performance liquid chromatography conditions: what the chromatographic column selects is Hypersil C18 250mm×4.6mm i.d., 7μm chromatographic column, mobile phase: acetonitrile containing zinc acetate 0.2mol, acetic acid 8.3mmol and 2.5v / v% in every liter of mobile phase, flow rate: 1.5ml / min; injection volume: 20μl, column temperature: 25°C, adjustable wavelength setting: 0-11min excitation wavelength 365nm, emission wavelength 480nm; 11-15.5min excitation wavelength 344nm, emission wavelength 404nm; 15.5-20min excitation light wavelength 254nm, emission light wavelength 404nm ( figure 1 ).
[0057] 2. Detection of serum samples
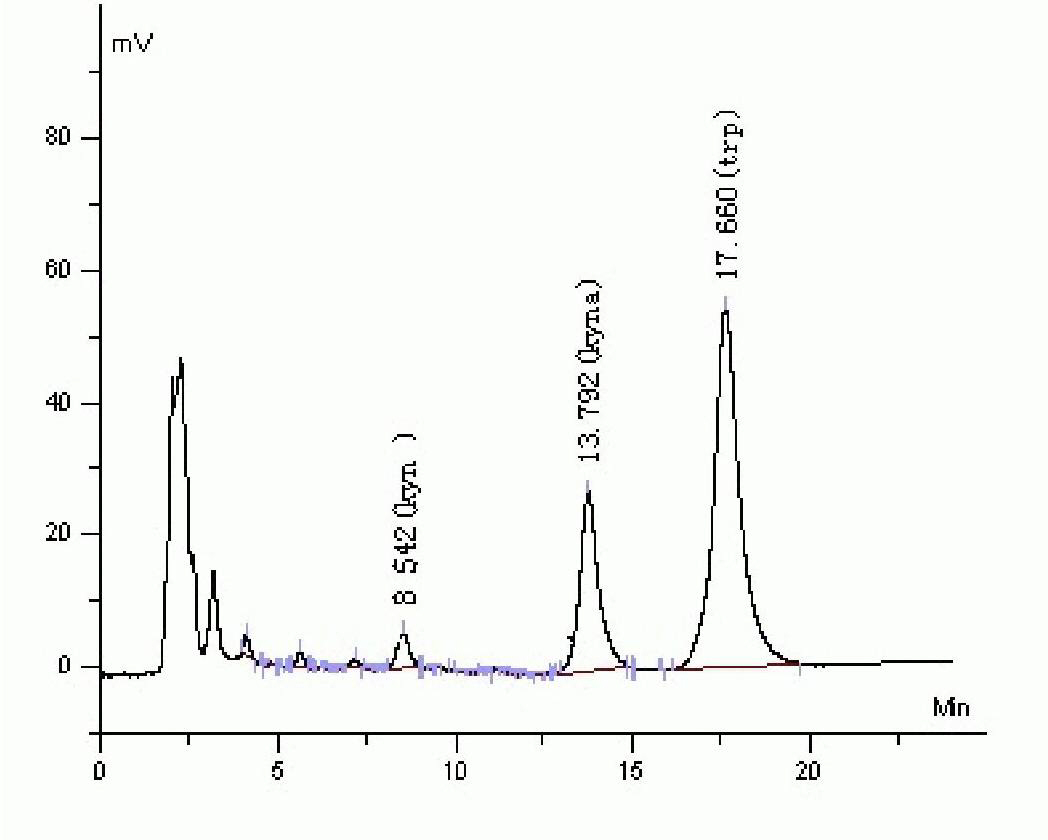
[0058] Take 200 μl of serum samples into an eppendorf tube, add an equal amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com