A kind of pharmaceutical composition containing ornidazole and its preparation
The technology of ornidazole and composition is applied in the field of pharmaceutical compositions containing ornidazole and preparations thereof, which can solve the problems of adverse reactions, easy to cause phlebitis, poor stability and the like, and achieves low impurity content and adverse reactions. Low incidence and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
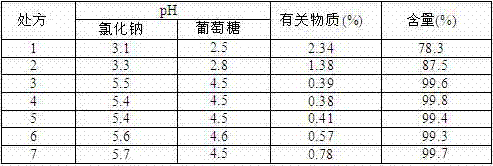
[0035] Prepare ornidazole injection according to the prescription in the table and the preparation process of the patent of the present invention:
[0036] Table 1 Prescription Screening Scheme
[0037]
[0038] Prepare ornidazole injection according to the method of the present invention: mix 1 / 2 amount of water for injection with propylene glycol; dissolve linoleic acid (not added in Example 1) in the above mixed solution; add ornidazole to the above obtained solution azole, stir, dissolve and make up to the total volume with water for injection; add 0.1% activated carbon for needles to the above obtained solution, stir for adsorption, and filter to remove carbon; fill the above obtained solution with ampoules; autoclave sterilization for injection , temperature 121 ℃, time 30min; light inspection, that is, 200 bottles.
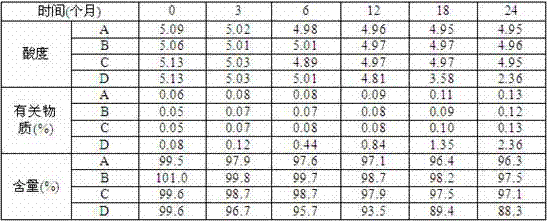
[0039] Under the packaging conditions of marketed drugs, they were stored for 3 months at a temperature of (25±2)°C and a relative humidity of 60%±10%,...
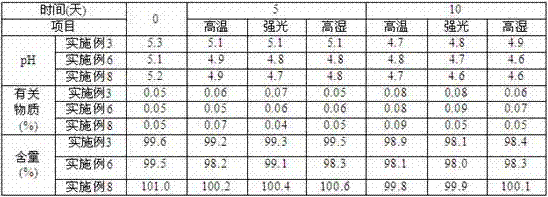
Embodiment 1
[0061] 1) Mix 1000ml water for injection with 10g propylene glycol;
[0062] 2) Dissolving 5g of linoleic acid in the above mixed solution;
[0063] 3) Add 100 g of ornidazole to the solution obtained above, stir, dissolve and dilute to 2000 ml with water for injection;
[0064] 4) Add 0.1% activated carbon for needles to the solution obtained above, stir for adsorption, and filter to remove carbon;
[0065] 5) Fill 400 bottles of the above-mentioned gained solution with ampoules;
[0066] 6) The injection is autoclaved at 121°C for 30 minutes;
[0067] 7) Light inspection, that is to say.
Embodiment 2
[0069] 1) Mix 1000ml water for injection with 20g propylene glycol;
[0070] 2) Dissolving 35g of linoleic acid in the above mixed solution;
[0071] 3) Add 200g of ornidazole to the solution obtained above, stir, dissolve and dilute to 2000ml with water for injection;
[0072] 4) Add 0.1% activated carbon for needles to the solution obtained above, stir for adsorption, and filter to remove carbon;
[0073] 5) Fill the above-mentioned gained solution with ampoules, totally 400 bottles;
[0074] 6) The injection is autoclaved at 121°C for 30 minutes;
[0075] 7) Light inspection, that is to say.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com