Substituted crotonamide maleate and crystal forms thereof
A technology of maleate and crotonamide, which is applied in the preparation of carboxylate, medical preparations containing active ingredients, and drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
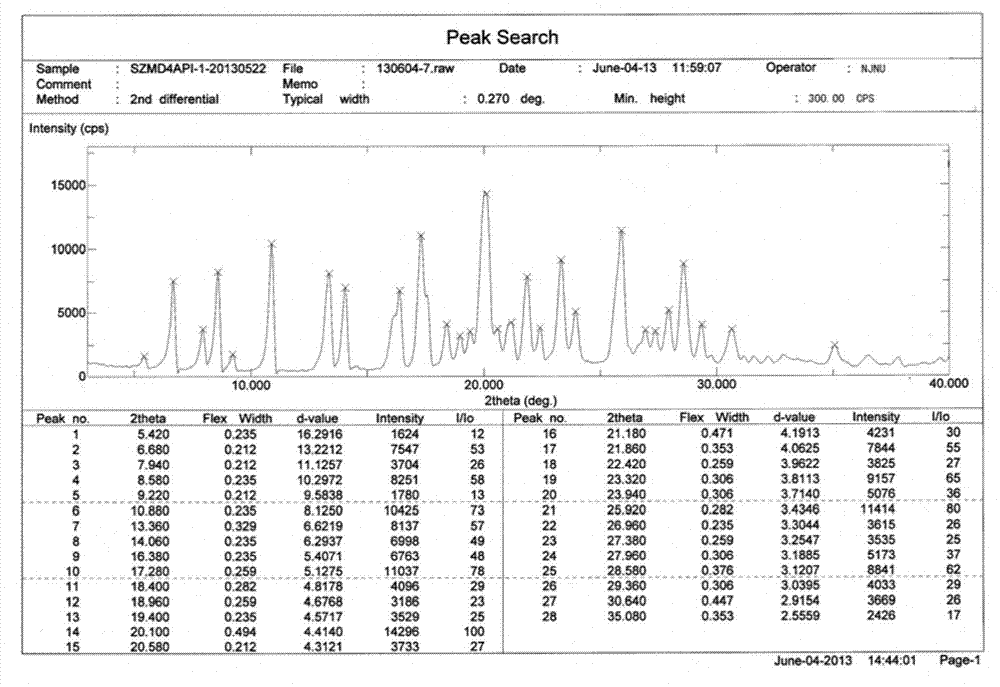
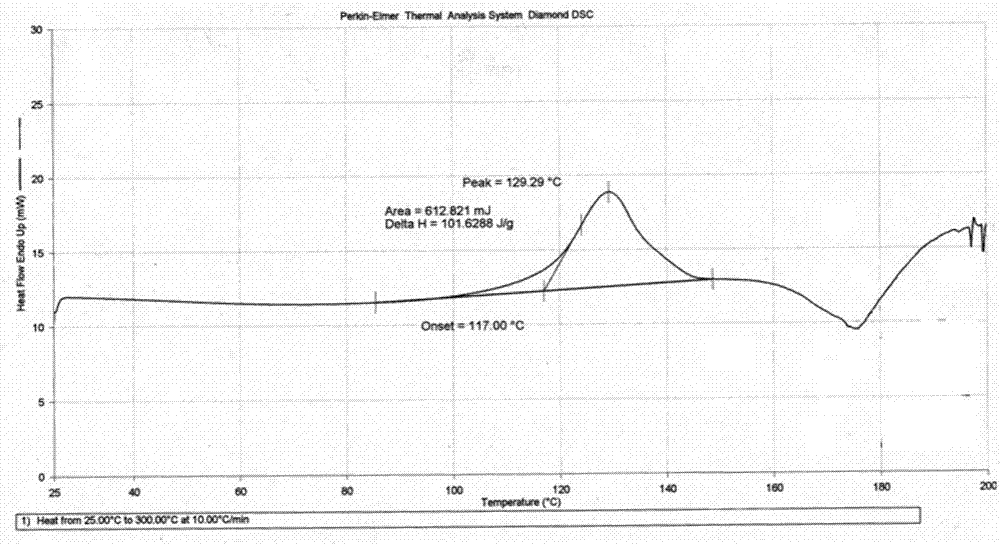
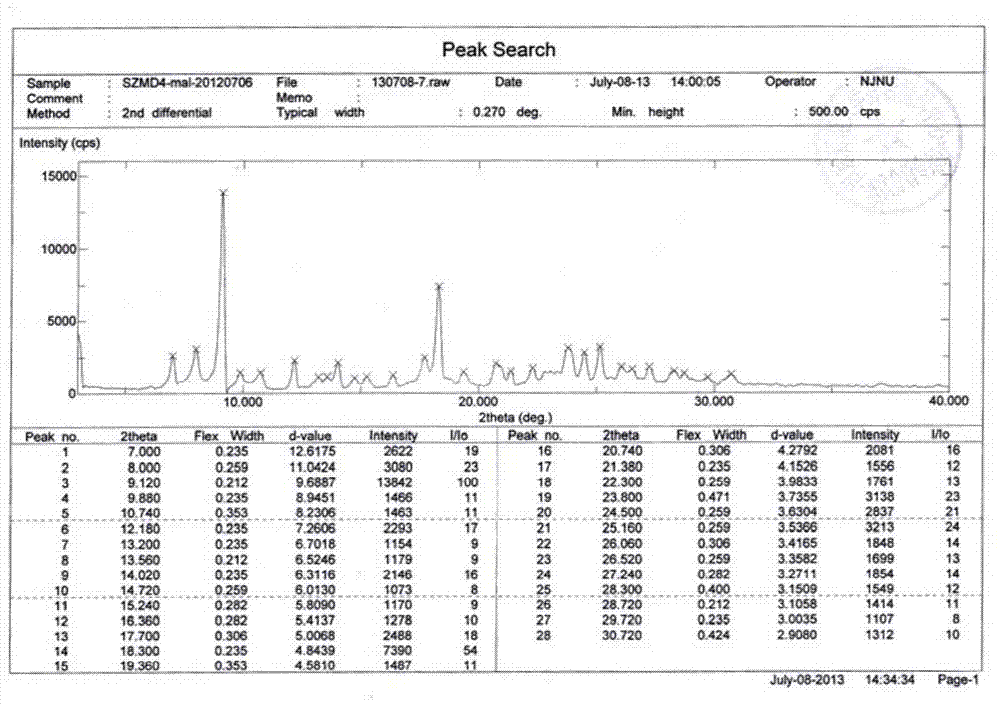
[0023](E)-N-{4-[(3-ethynylanilino)-3-cyano-7-ethoxy-6-quinolinyl]}-4-(dimethylamino)-2-butane Enamides are irreversible inhibitors of Her-2 (also known as ErbB-2 or neu) kinase. Her-2 is a member of the epidermal growth factor receptor (EGFR) family, and EGFR family members are closely related to the occurrence and development of tumors and the poor prognosis of tumor patients. (E)-N-{4-[(3-ethynylanilino)-3-cyano-7-ethoxy-6-quinolinyl]}-4-(dimethylamino)-2-butane The structure of the enamide free base is shown below:
[0024]
[0025] Compound (E)-N-{4-[(3-ethynylanilino)-3-cyano-7-ethoxy-6-quinolinyl]}-4-(dimethylamino)-2- Crotonamide is described in patent WO2010151710. According to the biopharmaceutical classification system (BCS), the compound is classified as class IV, low water solubility and poor permeability. At pH 7, the solubility of the free base in water is about 1 μg / ml. As the pH of the solution decreases, the solubility of the compound increases due to i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com