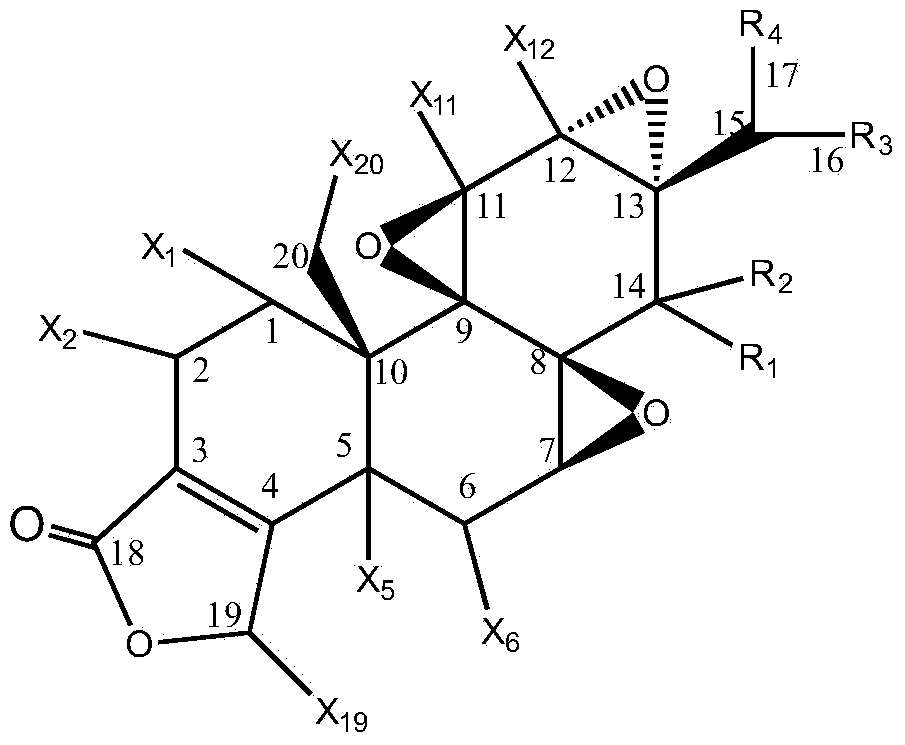
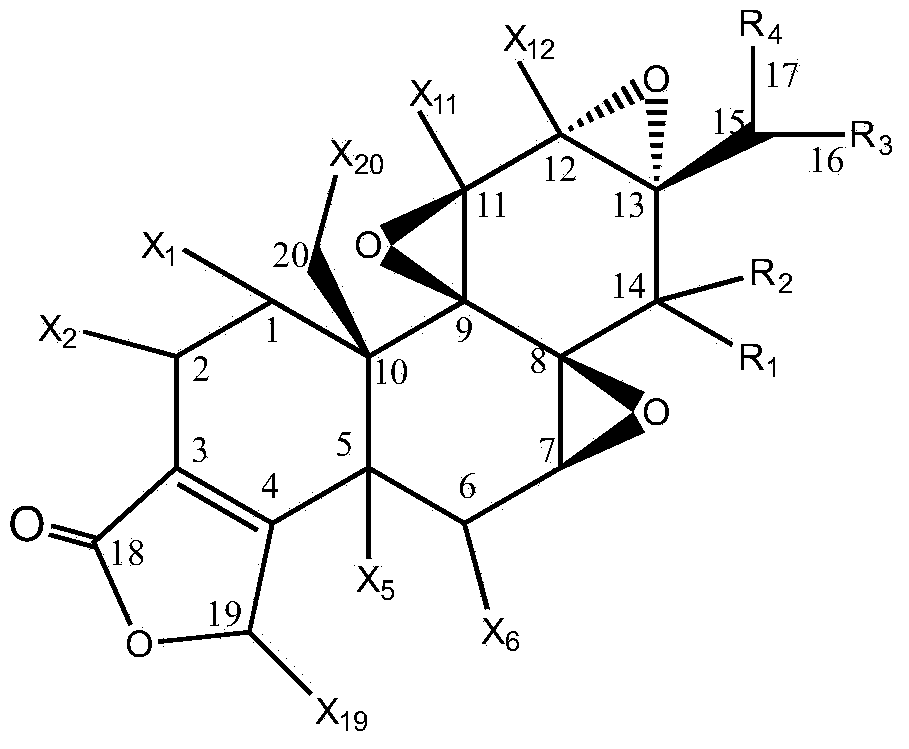
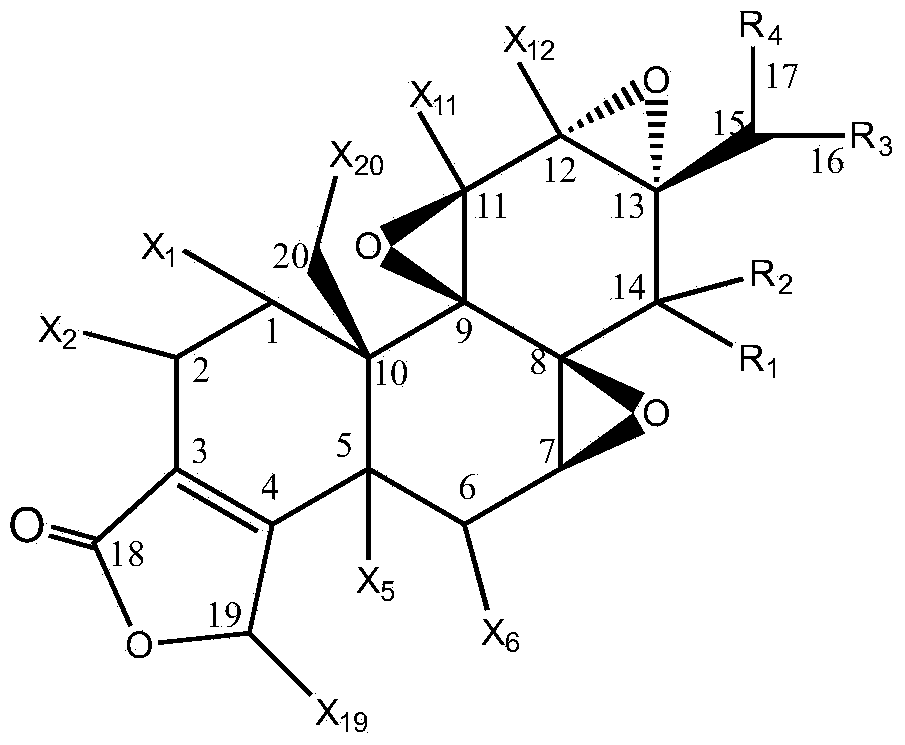
Preparation method of triptolide derivatives and products and application of triptolide derivatives
A technology for triptolide and derivatives is applied in the preparation of triptolide derivatives, products and application fields, and can solve the problems of low conversion rate, poor selectivity, low yield of chemical synthesis method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention relates to a kind of preparation method of triptolide derivative, comprising the following steps:
[0045] Step 1: Take a transformed strain stored at 4°C and cultivated on a potato slant medium, place it in a constant temperature incubator at 25°C for 7 days, and blow off the spores on the slope with an appropriate amount of sterile water to make a single Adjust the concentration of the spore suspension to 107-108 / mL to obtain the seed spore liquid;
[0046] Step 2: Inoculate the spore liquid obtained in step 1 on the potato liquid medium with an inoculum amount of 100ml and 5mL, and cultivate it for 12 hours at a pH of 6.0, a temperature of 30°C±1°C, and a shaking speed of 200r / min; press 0.1 ~4ug inoculum, add various combined elicitors in different proportions, continue to cultivate for 12 hours, then add triptolide according to the inoculum of 1~10ug, and then continue to cultivate for 5 days, detect various triptolide derivatives every day W...
Embodiment 1
[0080] Example 1: Preparation of Triptolide Derivatives
[0081] 1. Preparation of 6α-hydroxytriptolide
[0082] Inoculate 5mL (108 cells / mL) spore liquid of Cunninghamella blakesleana AS3.970 in 100mL potato liquid medium, under the condition of pH to 6.0, temperature 30℃±1℃, shaking speed 200r / min After incubation for 12 hours, add methyl jasmonate (MeJA, 0.35mmol / mL), dexamethasone (DXM) and β-cyclodextrose at a weight ratio of 0.2ug (3:2:1) (BCD, 0.51mmol / mL), continue to cultivate for 12 hours, then add triptolide according to the inoculum amount of 5%, then continue to cultivate for 5 days, stop the cultivation, and then centrifuge the filtrate at 8000r / min for 10min to remove the precipitate. The filtrate was extracted 3 times with an equal volume of ethyl acetate, and the extract was evaporated to dryness, dissolved in 2m L of acetone, and then separated by a chromatographic silica gel column. , 6:1, 4:1, 2:1, 1:1, 1:2, 1:4, 1:6, 1:8 petroleum ether: ethyl acetate, 1...
Embodiment 2
[0114] Embodiment two, pharmacodynamics evaluation experimental example
[0115] In the following experimental examples, the test sample is provided by the preparation method embodiment of the present invention, and the precursor compound triptolide is used as a positive control.
[0116] 1. The growth inhibitory effect of 12 compounds of the present invention on human lung cancer cell A549 cells cultured in vitro
[0117] Methods: Human A549 lung cancer cells were cultured in DMEM medium (Gibco, USA) containing 10% fetal bovine serum at 37°C, 5% CO 2 , tumor cells 0.7x10 4 / well was inoculated in a 96-well plate, and after 24 hours, the compound diluted with dimethyl sulfoxide (200uM) and PBS solution was added to make the final concentration of the culture medium 10 -4 、10 -5 、10 -6 、10 -7 、10 -8 M, after 72 hours of treatment, discard the culture medium, fix the cells with 10% cold trichloroacetic acid, stain with sulforhodamine B (SRB) solution, wash away the unbound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com