A class of gabab receptor negative allosteric modulator and its medical application
A technology for receptors and uses, applied in the field of ligand molecules for γ-aminobutyric acid B-type receptors, which can solve the problems of immaturity and lack of GABA negative allosteric regulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
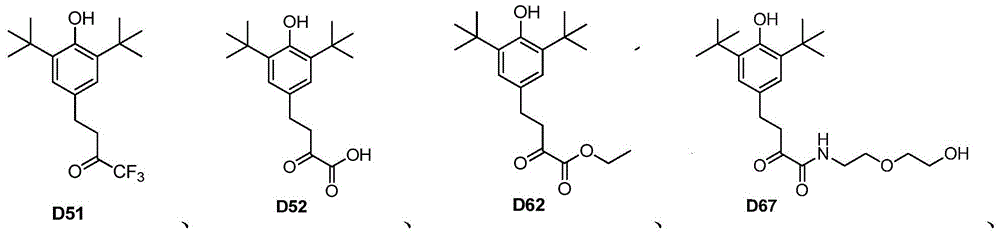
[0067] Preparation Example 1——Preparation of Compound D31
[0068]
[0069] Raw material aldehyde D1 (100mg, 0.427mmol, synthetic reference Chinese Journal of Medicinal Chemistry 2005,15,4) and Ph 3 After mixing P=CH-CHO (156mg, 0.512mmol, purchased from Bailingwei Reagent Company), add toluene (2ml), N 2 Under protection, heat at 100°C overnight. The reaction solution was cooled to room temperature, concentrated under reduced pressure, and subjected to silica gel column chromatography (PE / EA=40 / 1-10 / 1), to obtain D31 (19.2 mg, 17%) as a light reddish-brown solid. 1 H NMR (CDCl 3 ,300MHz):δ9.64(d,J=8.1Hz,1H),7.43(d,J=15.6Hz,1H),7.41(s,2H),6.62(dd,J=7.5Hz,15.6Hz,1H ),5.63(s,1H),1.46(s,18H).
preparation Embodiment 2
[0070] Preparation Example 2——Preparation of Compounds D73 and D60
[0071]
[0072] Preparation of compound D72
[0073] Raw materials aldehyde D1 (1.17g, 5.0mmol), triethyl orthoformate (4.1g, 38.6mmol), NH 4 Cl (0.05g, 0.935mmol) was mixed with MeOH (3ml) and heated to reflux for 3.5h. The reaction solution was gradually cooled to room temperature and concentrated under reduced pressure. The crude product was dissolved in DCM (10ml), diluted with PE60-90°C (20ml), a yellow solid precipitated, distilled at atmospheric pressure until the distillate was 60°C, and cooled to room temperature. The raw material was removed by filtration, and the filtrate was concentrated to obtain D72 (0.647 g, 46%) as a yellow solid. 1 H NMR (CDCl 3 ,300MHz): δ7.22(s,2H),5.25(s,1H),5.22(s,1H),3.35(s,6H),1.44(s,18H).
[0074] Preparation of compound E25
[0075] TMSCl (2.049g, 18.9mmol), DMAP (0.089g, 0.727mmol), and ethyl pyruvate (1.690g, 14.55mmol) were successively added to redistille...
preparation Embodiment 3
[0080]Preparation Example 3——Preparation of Compound D51
[0081]
[0082] Preparation of Compound D38
[0083] Raw material aldehyde D1 (0.234g, 1.00mmol) and malonic acid (0.310g, 3.0mmol) were mixed, under N2, redistilled pyridine (0.85ml) and piperidine (0.03ml) were added, and heated at 100°C for 1.5h. The reaction solution was poured into a mixture of 12N HCl (0.5ml) and ice water (0.8ml), stirred for 1h, and a large amount of orange solids precipitated out. Filter and wash the filter cake with water. After the filter cake was dissolved in DCM, PE was added at 60-90°C, and distilled at atmospheric pressure until the distillate was 60°C, and a white solid was precipitated. Cool to room temperature, filter, and wash the filter cake with PE to obtain milky white solid D38 (196 mg, 71%). 1H NMR (CDCl3, 300MHz): δ7.75(d, J=15.6Hz, 1H), 7.40(s, 2H), 6.31(d, J=15.6Hz, 1H), 5.56(s, 1H), 1.46( s,18H).
[0084] Preparation of Compound D53
[0085] D38 (507mg, 1.83mmol) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com