Homodimer for caffeic acid and ferulic acid, and preparation method and pharmaceutical composition thereof
A kind of drug and doublet technology, applied in the field of homodimer, can solve the problems that are not enough to delay the development of Alzheimer's disease and can't effectively treat neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
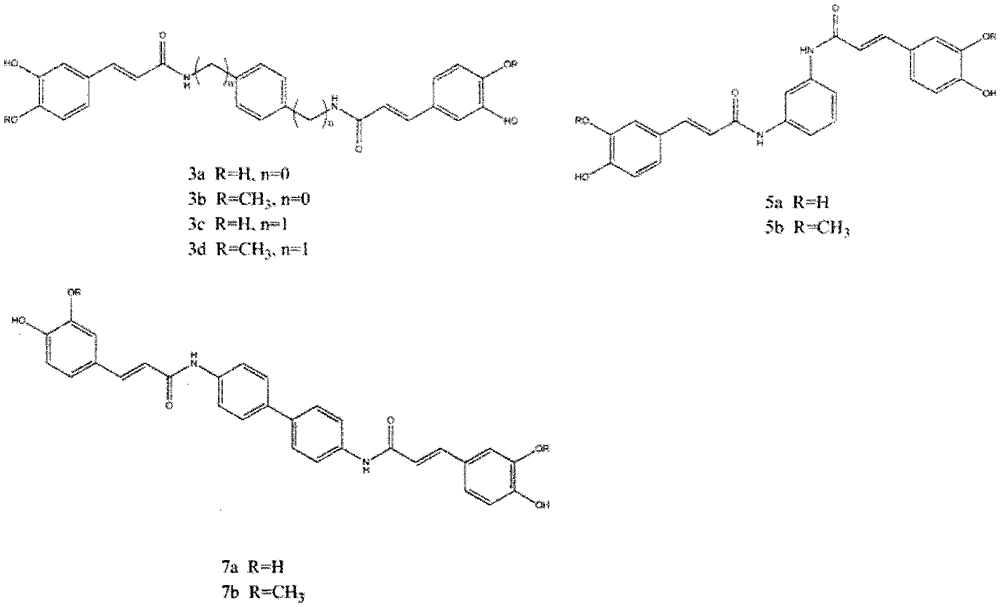
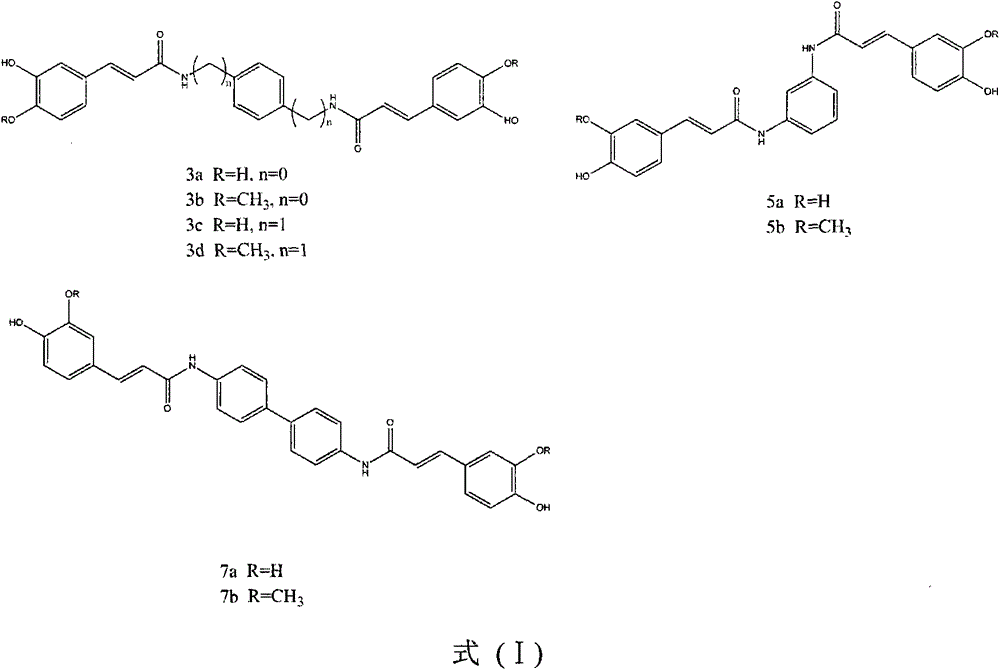
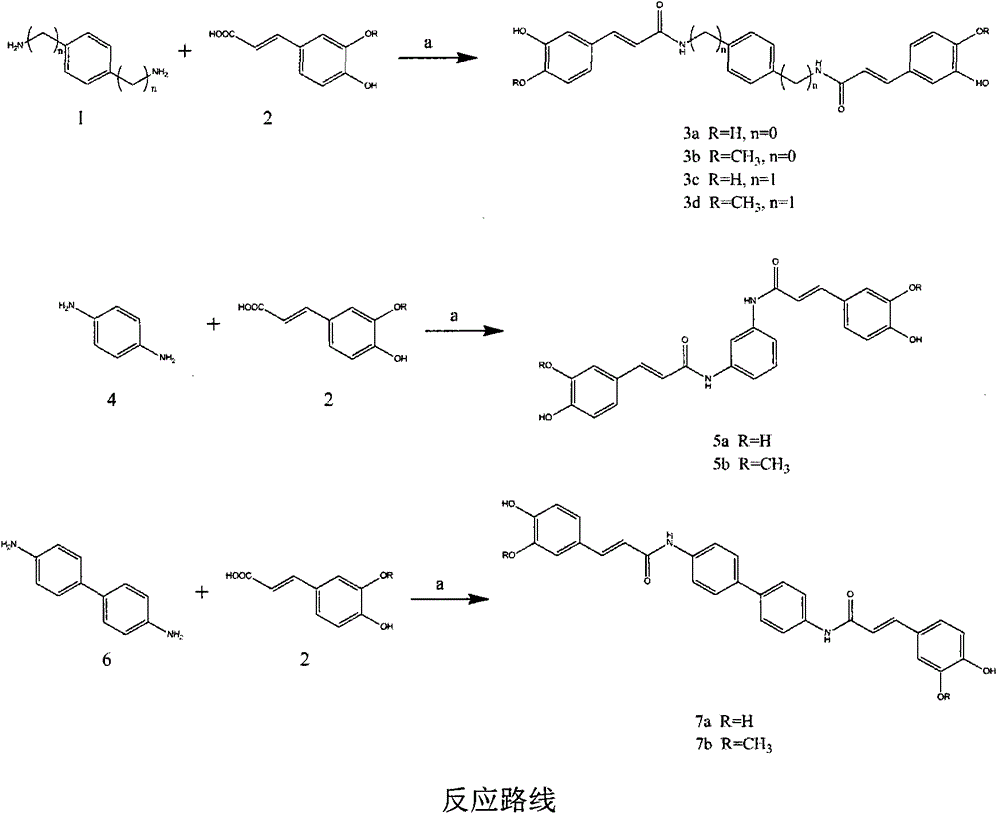
[0030] (E)-3-(3,4-dihydroxyphenyl)-N-{4-[3-(3,4-dihydroxyphenyl)-acrylamide]-phenyl}-acrylamide
[0031] Reagents: caffeic acid (166mg, 0.92mmol), p-phenylenediamine (50mg, 0.46mmol), BOP (407mg, 0.92mmol), treated according to the synthesis method of the general reaction formula to obtain a yellow solid with a yield of 21.4%.
[0032] 1 H-NMR (400MHz, DMSO-d 6 )d10.05(s, 2H), 8.32(s, 1H), 7.63(s, 4H), 7.38(d, J=15.5Hz, 2H), 7.01(s, 2H), 6.90(d, J=7.9 Hz, 2H), 6.78 (d, J = 8.1 Hz, 2H), 6.54 (d, J = 15.4 Hz, 2H).
[0033] 13 C NMR (100MHz, DMSO-d 6 )d 163.89, 148.68, 147.98, 140.51, 134.99, 126.37, 122.03, 119.66, 119.00, 115.82, 110.97.
Embodiment 2
[0035]
[0036] (E)-3-(4-Hydroxy-3-methoxyphenyl)-N-{4-[3-(4-hydroxy-3-methoxyphenyl)acrylamido]phenyl}-propene Amide
[0037] Reagents: ferulic acid (178mg, 0.92mmol), p-phenylenediamine (50mg, 0.46mmol), BOP (407mg, 0.92mmol), treated according to the general reaction formula to obtain a yellow solid with a yield of 30.2%.
[0038] 1 H NMR (400MHz, DMSO-d 6 )d10.06(s, 2H), 9.51(s, 2H), 7.65(s, 3H), 7.48(d, J=15.5Hz, 2H), 7.18(s, 2H), 7.11-7.00(m, 2H ), 6.83 (d, J=8.1 Hz, 2H), 6.64 (d, J=15.6 Hz, 2H), 3.83 (s, 6H).
[0039] 13 C NMR (100MHz, DMSO-d 6 )d 163.89, 148.68, 147.98, 140.51, 134.99, 126.37, 122.03, 119.66, 119.00, 115.82, 110.97, 55.66.
Embodiment 3
[0041]
[0042] (E)-3-(3,4-dihydroxyphenyl)-N-(4-{[3-(3,4-dihydroxyphenyl)-acrylamide]-methyl}-benzyl)-propene Amide
[0043] Reagents: caffeic acid (133.2mg, 0.74mmol), p-benzylidene diamine (50mg, 0.37mmol), BOP (325mg, 0.74mmol), processed according to the synthesis method of the general reaction formula to obtain a yellow solid with a yield of 25.6%.
[0044] 1 H NMR (400MHz, DMSO-d 6 )d8.45(s, 2H), 7.27(d, J=15.8Hz, 2H), 7.24(s, 4H), 6.94(s, 2H), 6.84(d, J=7.5Hz, 2H), 6.74( d, J = 8.0 Hz, 2H), 6.38 (d, J = 15.6 Hz, 2H), 4.35 (d, J = 4.6 Hz, 4H).
[0045] 13 C NMR (100MHz, DMSO-d 6 )d165.50, 147.40, 145.58, 139.54, 138.17, 127.45, 126.41, 120.51, 118.35, 115.82, 113.92, 42.09
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com