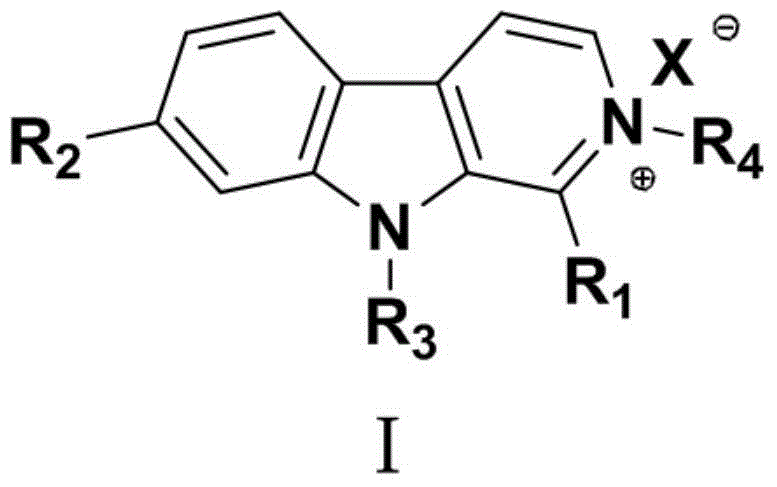
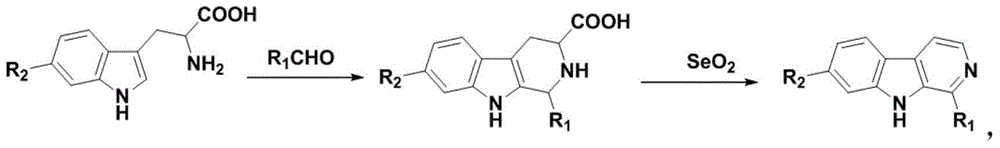
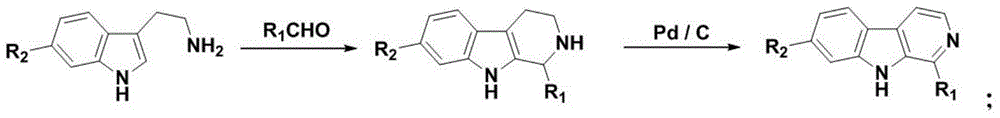
2-substituted-beta-carboline compounds and application thereof in preparing drugs for preventing or treating tumors
A compound, carboline technology, applied in the field of preparation of 2-substituted β-carboline compounds, can solve the problems of tumor treatment failure and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment is the synthesis of 1-methyl-2-benzyl-7-methoxy-9-octyl-β-carboline chloride salt (compound 1):
[0042]
[0043] Add 1mmol of 1-methyl-7-methoxy-9-octyl-β-carboline into a 50mL round bottom flask, add 25mL of ethyl acetate and 2mmol of benzyl chloride, and react at 80°C for 24h; , separated by silica gel column chromatography to obtain 1-methyl-2-benzyl-7-methoxy-9-octyl-β-carboline chloride salt (1) (0.34 mmol, yield 34%). 1H-NMR (400MHz, MeOD): 8.65(d, J=6.6Hz, 1H), 8.48(d, J=6.6Hz, 1H), 8.30(d, J=8.7Hz, 1H), 7.40-7.49(m ,3H),7.21-7.24(br,3H),7.12(dd,J=2.1,8.8Hz,1H),6.04(s,2H),4.69(t,J=7.6Hz,2H),4.69(s, 3H), 3.32-3.34(m, 2H), 1.83-1.90(m, 2H), 1.26-1.42(m, 10H), 0.88(t, J=6.87Hz, 3H); 13C-NMR(400MHz, MeOD) :δ164.64,148.23,135.35,134.31,134.17,129.24(2C),128.50,126.44(2C),124.03,113.86,113.42,113.05,93.14,60.37,55.26,45.44,31.53,30.06,28.94,28.83,26.24,22.21 ,13.05; ESI-MS m / z:451.2[M+H] + .
Embodiment 2
[0045] This embodiment is the synthesis of 1-methyl-2-benzyl-7-methoxy-9-allyl-β-carboline chloride salt (compound 2):
[0046] With reference to the synthetic route shown in Example 1, 1mmol of 1-methyl-7-methoxy-9-allyl-β-carboline was added to a 50mL round bottom flask, and 25mL of ethyl acetate and 2mmol of chlorine were added Benzyl chloride, reacted at 80°C for 24h; after reducing the concentration, silica gel column chromatography separated to obtain 1-methyl-2-benzyl-7-methoxy-9-allyl-β-carboline chloride salt ( 2) (0.35 mmol, 35% yield). 1 H-NMR (400MHz, MeOD): 8.65(d, J=6.6Hz, 1H), 8.49(d, J=6.6Hz, 1H), 8.32(d, J=8.7Hz, 1H), 7.45-7.40(m ,3H),7.21(d,J=6.94Hz,1H),7.15(d,J=5.15Hz,1H),7.13(d,J=8.7Hz,1H),6.02(s,2H),5.33(m ,2H),5.28(d,J=10.62Hz,1H),5.56(s,3H),4.01(s,3H); 13 C-NMR (MHz, MeOD): 164.82, 148.35, 139.14, 135.86, 135.55, 134.20, 134.11, 133.25, 129.17 (2C), 128.65, 126.43 (2C), 124.10, 115.58, 114.03, 113.407, 1903 ,55.21; ESI-MS m / z:379.3[M+H] +.
Embodiment 3
[0048] This example is the synthesis of 1-methyl-2-benzyl-7-methoxy-9-(4-methyl)benzyl-β-carboline chloride salt (compound 3):
[0049] Referring to the synthetic route shown in Example 1, 1mmol of 1-methyl-7-methoxy-9-(4-methyl)benzyl-β-carboline was added to a 50mL round bottom flask, and 25mL of acetic acid was added Ethyl ester and 2mmol benzyl chloride were reacted at 80°C for 24h; after reducing the concentration, silica gel column chromatography separated to obtain 1-methyl-2-benzyl-7-methoxy-9-(4-methyl) Benzyl-β-carboline chloride salt (3) (0.41 mmol, 41% yield). 1 H-NMR (400MHz, MeOD): 8.66(d, J=6.6Hz, 1H), 8.53(d, J=6.6Hz, 1H), 8.37(d, J=8.8Hz, 1H), 7.37-7.41(m ,3H),7.19(d,J=9.78Hz,1H),7.12-7.17(m,5H),6.90(d,J=8.0Hz,2H),5.96(s,2H),5.94(s,2H) ,3.95(s,3H),2.93(s,3H),2.30(s,3H); 13 C-NMR(400MHz,MeOD):164.89,148.92,139.48,137.61,135.90,135.74,134.55,134.22,133.76,129.58,129.08,128.55,126.30,125.04,124.23,114.09,113.84,113.11,92.86,60.33,55.25 ,48.50,19.64,15.27; ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com