Regorafenib and manufacture method thereof
A compound and monohydrate technology, applied in the field of preparation of regorafenib and regorafenib, can solve problems such as excessive vascular proliferation and blindness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
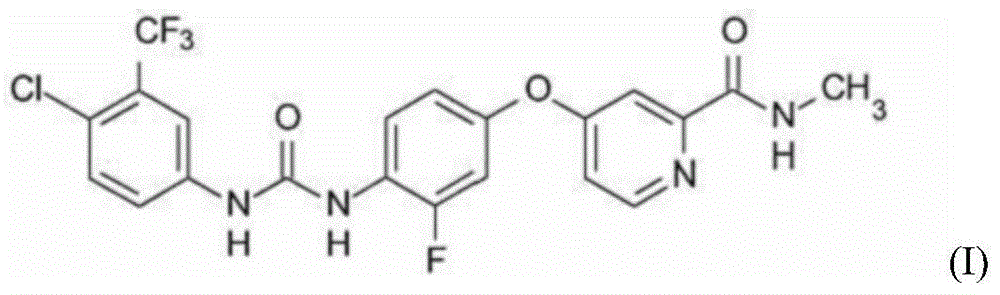
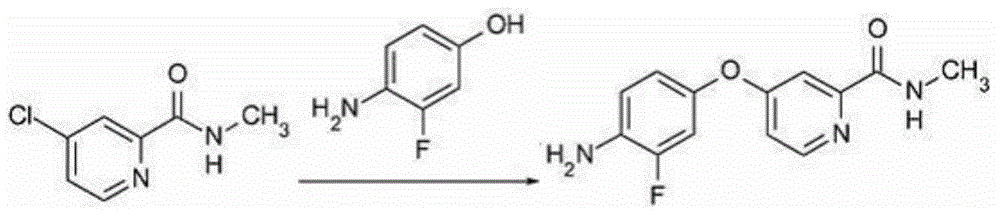
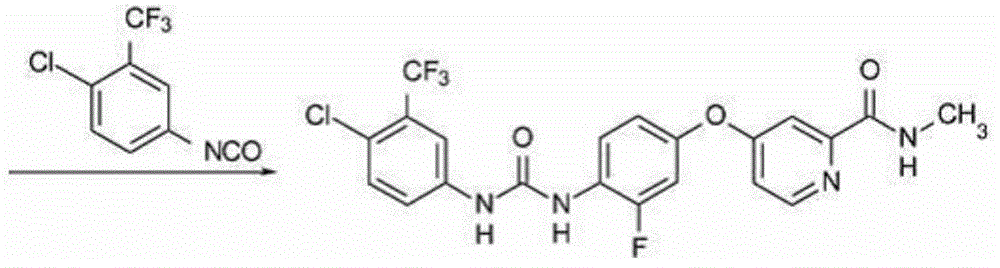
[0216] Example 1: 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy}-N-methylpyridine- 2-carboxamide, Preparation of its hydrochloride and its monohydrate
[0217] Phase 1:
[0218] 4-chloro-N-methyl-pyridine-2-carboxamide hydrochloride:
[0219] 420 g of 4-chloro-N-methylpyridine-2-carboxamide (prepared according to WO2006 / 034796) in toluene (approximately 30% w / w) and 48.8 g of ethanol were added to the reaction flask. 67.2 g of acetyl chloride was added under stirring so that the temperature of the reaction mixture did not exceed 30°C. After further stirring for 1.5 h at room temperature, the product was filtered off, washed with toluene (212 g) and dried under reduced pressure (30° C., 80 mbar). In this way, 156.3 g (quantitative yield) of 4-chloro-N-methyl-pyridine-2-carboxamide hydrochloride was obtained. The melting point is 173.7-174.5°C.
[0220] 1 H-NMR(500MHz, DMSO-d 6 ): δ [ppm] = 2.96 (d, 3H), 7.79-7.97 (m, 1H), 8.13-8.26 (m, 1H), 8...
Embodiment 2
[0269] Example 2: Investigation of the stability of the bulk drug
[0270] Respectively, the various purity of the compound of formula (I) monohydrate and the compound of formula (I) anhydrate prepared in the above embodiment 1, its various stages, and each supplementary test example were prepared and composited Film sealed packaging, placed in a 45℃ thermostat for 4 months for high temperature treatment. Determine the purity and relative content of the compound of formula (X) of each drug substance at month 0 and April, and compare the purity of each batch of samples with the relative content of the compound of formula (X). The percentage change in content characterizes this change. The calculation formula of the two parameters is as follows:
[0271] Purity change value = 0 month purity-4 month purity
[0272] Percentage of relative content change=[(relative content in April-0 relative content in April)÷relative content in 0month]×100%
[0273] The results show that:
[0274] The...
Embodiment 3
[0275] Example 3: Preparation of pharmaceutical composition in tablet form
[0276] With reference to the formula and preparation method of steps a), b), and c) of Example 1 on page 19 of WO2014039677A1, the compound of formula (I) monohydrate or compound of formula (I) prepared in Example 1 of the present invention is used respectively. The medicinal raw material of Aquatic is the active ingredient, and coated tablets are prepared (each tablet contains 40 mg of the compound of formula (I)).
[0277] Each of the obtained tablets was sealed and packaged with an aluminum-plastic composite film, and placed in a 45°C thermostat for 4 months for high-temperature treatment. With reference to the method in Example 2 above, determine the content of the compound of formula (I) and the content of the compound of formula (X) relative to the compound of formula (I) in each tablet at month 0 and April, and compare each tablet respectively The change value of the residual content of the compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com