Antitumor drugs and preparation method and application thereof
A drug and pharmaceutical technology, applied in the field of regorafenib, can solve problems such as blindness and excessive blood vessel proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
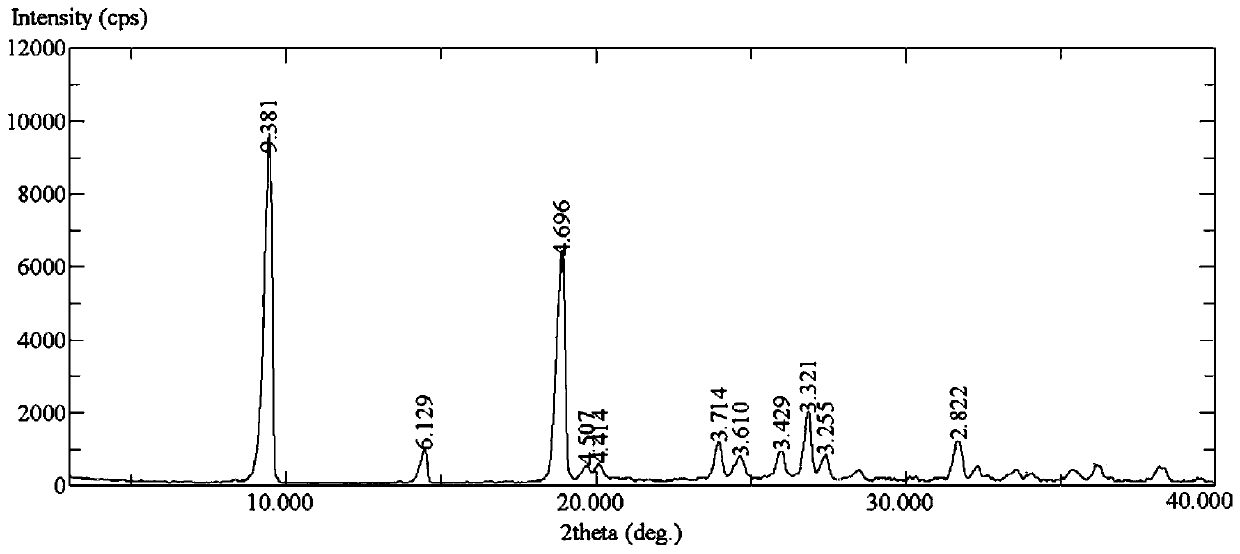
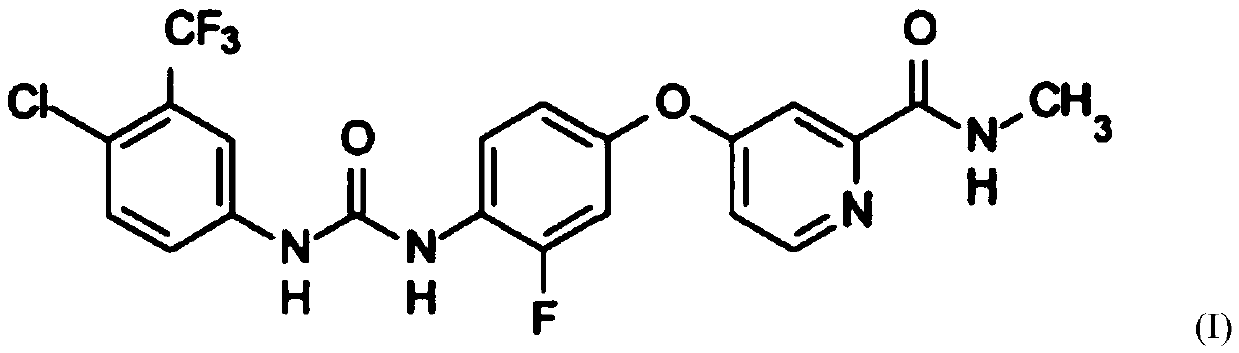
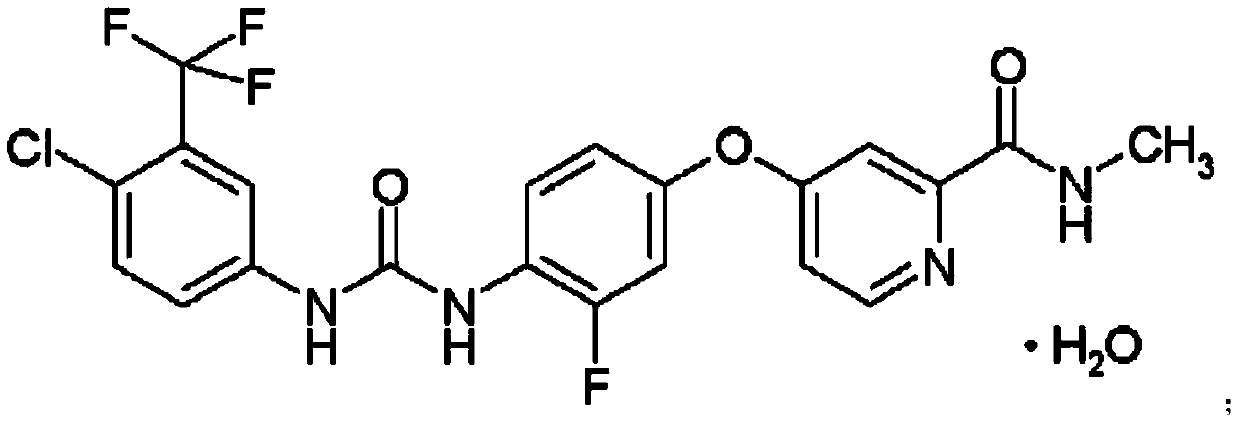
[0098] Example 1: 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy}- Preparation of N-picoline-2-carboxamide, its hydrochloride and its monohydrate
[0099] Embodiment 1 of the present invention is basically carried out with reference to the method of Embodiment 1 of CN104592105A.
[0100] Phase 1: Preparation of 4-chloro-N-methyl-pyridine-2-carboxamide hydrochloride
[0101] 420 g of a solution (approximately 30% w / w) of 4-chloro-N-methylpyridine-2-carboxamide (prepared according to WO2006 / 034796) in toluene and 49 g of ethanol were added to the reaction flask. 67.5 g of acetyl chloride was added with stirring to such an extent that the temperature of the reaction mixture did not exceed 30°C. After further stirring at room temperature for 1.5 h, the product was filtered off, washed with toluene (210 g) and dried under reduced pressure (30° C., 80 mbar). In this way, 156.0 g (quantitative yield) of the product 4-chloro-N-methyl-pyridin...
Embodiment 2
[0122] Example 2: Preparation of 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy Base}-N-methylpyridine-2-carboxamide (anhydrous)
[0123] 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy}- N-picoline-2-carboxamide monohydrate was dried under reduced pressure at 90°C (21mbar) for 3 hours, then vacuumized, and then filled with a mixed gas of carbon dioxide and nitrogen at a volume ratio of 60:40 into the desiccator , sealed, and continue to keep the drier at this 90°C for 2 hours, then drop to room temperature at a cooling rate of 1°C / min, and then maintain it in the above-mentioned mixed atmosphere for 2 hours to obtain 4.67g of white crystalline solid as 4-{4 -[({[4-Chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)-amino]-3-fluorophenoxy}-N-methylpyridine-2-carboxamide. The melting point of the product is 212.5-213.0° C., and the 1H-NMR and MS data are consistent with the anhydrous product obtained in S...
Embodiment 3
[0131] Example 3: Preparation of 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy Base}-N-methylpyridine-2-carboxamide (anhydrous)
[0132] 4-{4-[({[4-chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)amino]-3-fluorophenoxy}- N-methylpyridine-2-carboxamide monohydrate was dried under reduced pressure (21mbar) at 90°C for 3 hours, then vacuumized, and then filled the desiccator with a mixed gas of carbon dioxide and nitrogen at a volume ratio of 55:45 , sealed, and continue to keep the drier at this 90°C for 2 hours, then drop to room temperature at a cooling rate of 1°C / min, and then maintain it in the above-mentioned mixed atmosphere for 2 hours to obtain 4.68g of white crystalline solid as 4-{4 -[({[4-Chloro-3-(trifluoromethyl)-phenyl]amino}carbonyl)-amino]-3-fluorophenoxy}-N-methylpyridine-2-carboxamide. The melting point of the product is 211.8-212.6° C., and the 1H-NMR and MS data are consistent with the anhydrous product obtained in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com